Using Fictional Elements for Chemistry Instruction
Steven Murov
Professor Emeritus of Chemistry, Modesto Junior College
Modesto, CA
Films and the literature[1]
abound with descriptions and applications of make-believe elements, compounds
and mixtures. In one instance,
Donald Duck gave a formula for methylene before the existence of methylene had
been confirmed.[2]
An extensive list of fake materials[3]
has been posted along with at
least three “periodic”
tables of fake materials.[4]
Fictional materials with educational applications have been
discussed.[5]
Although fictional, these materials provide stimulating material that
could be useful for pedagogical purposes.
While these fictional materials include such intriguing names as
unobtainium, afraidium (a bright yellow metal that tastes like chicken)3a,
bolognium, bombastium, and
jumbonium3, this article will focus on only three of fake materials:
dilithium, kryptonite and vibranium.
Descriptions of the properties of these make-believe materials are
notable3 but include minimal quantitative data.
However, it is still possible to create interesting questions for
students that can be used to increase understanding of chemistry concepts.
1. Granted that these materials are
fictional, is it still appropriate to change or misuse the meaning of chemistry
terms such as element and isotope to characterize these substances?
2. The fictional materials have been arranged in “Periodic” tables.4
a. Explain why the word periodic is a part of the title of the traditional table
of elements.[6]
b. Is it appropriate to use the word “periodic” in the titles of tables4
of fictional substances?
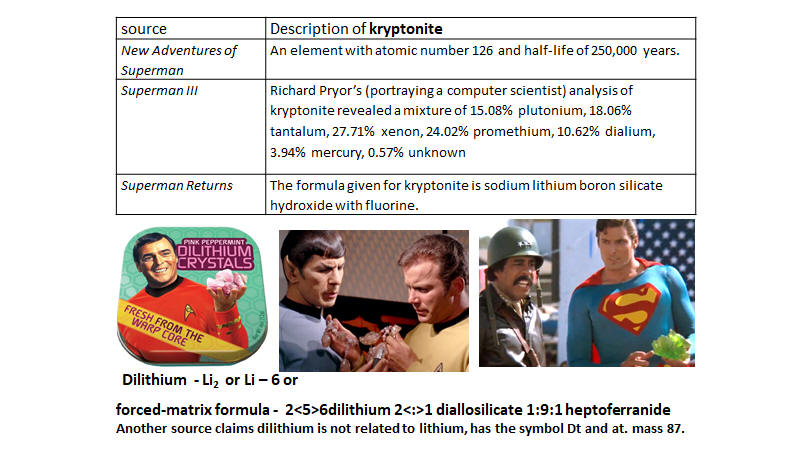
3. In Star Trek, the starship
Enterprise is said to be powered by dilithium crystals.
a. Using atomic orbitals, explain why the halogens, hydrogen, nitrogen and
oxygen are diatomic.
b. Considering the atomic orbitals of lithium, would you expect lithium to be
diatomic (Li2) in the gas phase?
c. According to the Internet, are lithium and/or iodine diatomic in the solid
state?
d. Nuclear fusion research is ongoing.
Some of the proposed reactions utilize an isotope of lithium – lithium 6.[7]
Is it a stretch to think dilithium refers to lithium-6?[8]
e. Is it conceivable that nuclear fusion could propel the Enterprise to greater
than light speed?
f. The Warp drive apparently does not use nuclear fusion but employs a matter –
antimatter reactor that utilizes dilithium.
Could this reactor provide Warp speed?
g. According to the literature, dilithium is
2<5>6dilithium 2<:>1 diallosilicate 1:9:1 heptoferranide.[9]
Another source claims dilithium is not related to lithium and has the
symbol Dt and an atomic mass of 87.
Do these claims make this discussion a moot point?
4. In Superman, kryptonite is
supposed to be Superman’s Achilles’ heel rendering him basically powerless but
kryptonite is harmless at least short term to humans.
Most commonly it is described as a
green crystal but many colored forms with differing properties have been
reported.10 Superman
stories contain three very different formulations for kryptonite:
|
source |
Description of kryptonite |
|
New Adventures of Superman |
A material with atomic number 126 and half-life of 250,000 years.[10] |
|
Superman III |
Richard Pryor’s (portraying a computer scientist) analysis of kryptonite
revealed a mixture of 15.08% plutonium, 18.06% tantalum, 27.71% xenon,
24.02% promethium, 10.62% dialium,[11]
3.94% mercury, 0.57% unknown.
|
|
Superman Returns |
The formula given for kryptonite is sodium lithium boron silicate
hydroxide with fluorine.[12]
|
a. Does it make sense for kryptonite to have different formulations?
b. Is it possible that kryptonite can have different colors?[13]
c. Would the “Pryor” kryptonite be harmless to humans?
d. Is the suffix ite in kryptonite appropriate for any of the three
formulations?
e. Is a half-life of 250,000 years appropriate for an element with atomic number
126?[14]
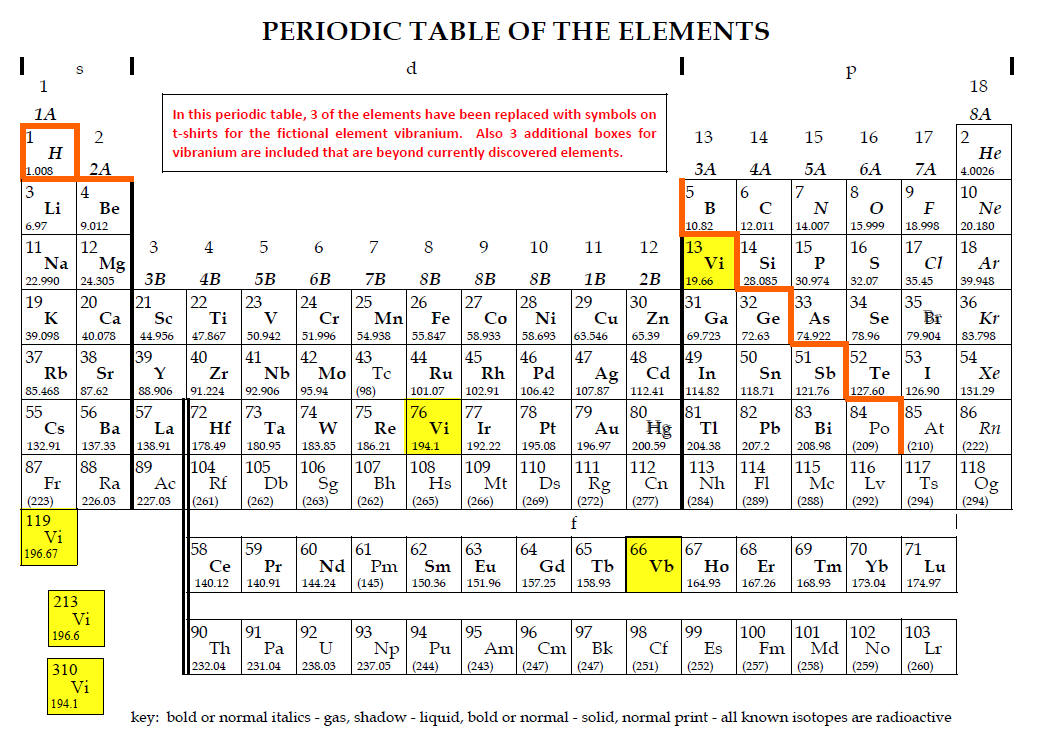
5. Several Marvel productions
including the recent Black Panther
discuss substantial use of the “element” vibranium.
Two “ isotopes” that have sharply
contrasting properties have been described.3,[15]
While the movies do not apparently
assign atomic number and mass values to vibranium, t-shirt companies have taken
the liberty to assign the values[16]
below:
|
Vibranium atomic number |
Atomic mass (g/mol) |
|
13 |
19.66 |
|
66 |
Not given |
|
76 |
194.1 |
|
119 |
196.67 |
|
213 |
196.6 |
|
310 |
194.1 |
a. Do isotopes generally have significantly different chemical, physical or
nuclear properties?
b. Critically evaluate (consider proton to neutron ratios and compare to
periodic table values) each of the values.
Two of the atomic numbers appear to have impossible atomic mass values.
Explain.
c. There is some consistency above with the values of atomic mass.
The four similar values yield an average atomic mass of 195.3.
Which, if any, of the atomic numbers makes the most sense for an atomic
mass of 195 and why?
d. Where should vibranium be placed in the periodic table?
e. The 196 values indicate isotopes exist[17]
but the 194.1 values also are consistent with isotopes (consider the atomic mass
of gold). Explain these claims.
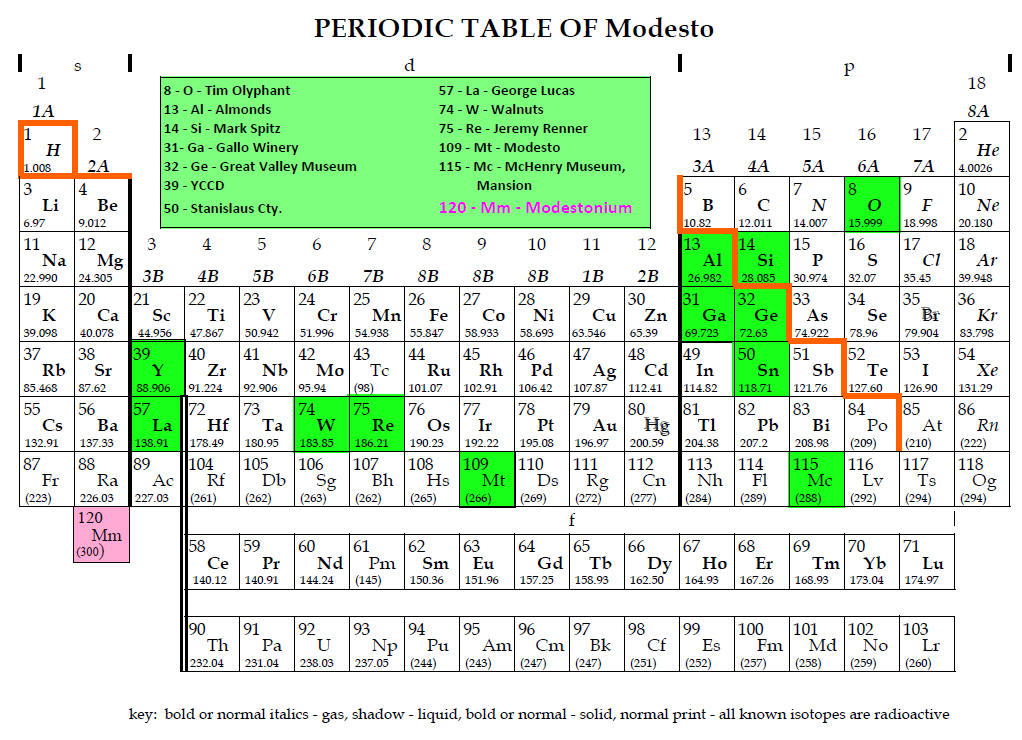
Admittedly, this author has also contributed to the lore of make-believe
elements. In an early edition
of a lab book, a problem that involved an atomic mass calculation of the element
Modestonium (Mt) was included.[18]
However, a few years later, element 109
was discovered and named Meitnerium and assigned the symbol Mt in honor of the
great theoretical chemist, Lise Meitner.
As a result, the symbol in the atomic mass calculation problem was
changed to Mm[19]
(an element with a colorful electron shell and a tasty nucleus) in later
editions.
In addition to providing interesting class and homework exercises, make-believe materials are well suited for exhibits. Suggestions for use as a part of a several station chemistry exhibit[20] have been published.


[16] For example, using Google
images, search for “Vibranium symbol.”
[17] S. Murov,
Chem 13 News, March,
2010.
“Promoting Insight:
Atomic Mass.”
[18] S. Murov and B. Stedjee,
Experiments in Basic Chemistry,
Wiley, 3rd ed., 1994, p 275.
[19] a. S. Murov and B. Stedjee,
Experiments and Exercise in Basic
Chemistry, Wiley, 7th ed.,
2009, p 317
b.
http://exercises.murov.info/ex3-2.htm problem 32.
[20] a. S. Murov and A. Chavez,
J. Chem. Educ.,
2017,
94 (10), pp 1571–1579,
b.
http://murov.info/EM-TOC.htm