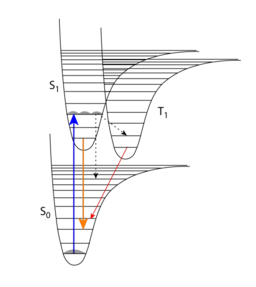
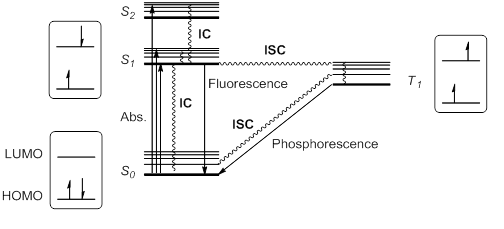
 Photophysical
Properties of Photosensitizers and Quenchers
Photophysical
Properties of Photosensitizers and Quenchers
 Photophysical
Properties of Photosensitizers and Quenchers
Photophysical
Properties of Photosensitizers and Quenchers
For an addendum to this site with data not included below, please visit:
http://murov.info/murovthesis.pdf , Steven Murov, murovs@yosemite.edu
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| a-1 | acenaphthene | n | C12H1O | 154.2 | 374 | 46 | 0.50 | 0.46 | 250 | ||||||
| p | 376 | 0.39 | 0.57 | 0.58 | 0.23 | 248 | 0.05 ? | 3300 | 2.6 | ||||||
| a-2 | acetone | n | C3H6O | 58.08 | 1.7 | 0.0009 | 0.90 | 6.3 | |||||||
| p | 2.1 | 0.01 | 1 | 332 | 0.042 | 47 | 0.0006 | ||||||||
| a-3 | acetophenone | n | C8H8O | 120.15 | 330 | <10-6 | 1 | 0.90 | 310 | 0.65 | 0.23 | 0.0021 | |||
| p | 338 | 0.0 | 1 | 311 | 0.60 | 0.14 | 0.0050 | ||||||||
| a-4 | - 4-methoxy | n | C9H10O2 | 150.18 | 300 | 0.35 | |||||||||
| p | 299 | 0.72 | 0.26 | ||||||||||||
| a-5 | acridine | n | C13H9N | 179.13 | 0.045 | 0.0001 | 0.5 | 0.15 | 190 | 104 | |||||
| p | 315 | 0.35 | 0.0079 | 0.82 | 188 | 0.155 | 14 | ||||||||
| a-6 | 9(10H)-acridinone | n | C13H9NO | 195.22 | 304 | 0.78 | 12.9 | 0.015 | 0.99 | 244 | 0.014 | 20 | |||
| p | 290 | 10.8 | 0.97 | 0.78 | 252 | 9.2 | 2.5 | ||||||||
| a-7 | aniline | n | C6H7N | 93.13 | 398 | 6.9 | 0.17 | 0.17 | 0.75 | 297 | 0.65 | 0.72 | 4.2 | ||
| p | 384 | 16.6 | 0.10 | 0.21 | 0.90 | 321 | 0.67 | 4.0 | |||||||
| a-8 | - N,N-dimethyl | n | C8H11N | 121.19 | 383 | 2.4 | 0.11 | 0.87 | |||||||
| p | 375 | 2.8 | 317 | ||||||||||||
| a-9 | - N,N-diphenyl | n | C18H15N | 245.32 | 362 | 0.045 | 0.06 | ||||||||
| p | ? 1.1 | 291 | 0.74 | ||||||||||||
| a-10 | - N-phenyl | n | C12H11N | 169.23 | 0.05 | 0.32 | 0.3 | ||||||||
| p | 372 | 2.4 | 2.6 | 0.11 | 0.12 | 0.47 | 301 | 0.87 | 0.5 | 1.9 | |||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| a-12 | anthracene | n | C14H10 | 178.23 | 318 | 5.3 | 0.30 | 0.71 | 178 | 670 | |||||
| p | 319 | 5.8 | 6.5 | 0.27 | 0.34 | 0.66 | 178 | 0.0003 | 3300 | ~0.04 | |||||
| a-13 | - 9,10-diphenyl | n | C28H18 | 330.43 | 304 | 7.7 | 7.9 | 0.91 | 0.02 | 171 | 2500 | ||||
| p | 305 | 8.2 | 7.95 | 0.95 | 1.0 | 0.02 | 3000 | ||||||||
| a-14 | 9,10-anthraquinone | n | C14H8O2 | 208.22 | 284 | 0.90 | 261 | 0.11 | |||||||
| p | 263 | 0.29 | |||||||||||||
| a-15 | azulene | n | C10H8 | 128.17 | 170 | 1.4 S2 | 0.02 S2 | 163 | 11 | ||||||
| p | 301 | 3 | |||||||||||||
| b-1 | benzaldehyde | n | C7H6O | 106.12 | 323 | <10-6 | 0 | 298 | |||||||
| p | 0.49 | ||||||||||||||
| b-2 | benz[a]anthracene | n | C18H12 | 228.29 | 311 | 45 | 0.19 | 0.79 | 197 | 100 | |||||
| p | 307 | 40.0 | 0.22 | 0.79 | 198 | 9400 | |||||||||
| b-3 | benzene | n | C6H6 | 78.11 | 459 | 34 | 0.06 | 0.17 | 0.25 | 353 | 0.15 | 4.5 | |||
| p | 459 | 28 | 0.04 | 0.19 | 0.15 | 353 | 0.18 | 6.3 | |||||||
| b-4 | - d6 | n | C6D6 | 84.15 | 449 | 30 | 0.042 | 0.25 | |||||||
| p | |||||||||||||||
| b-5 | -chloro | n | C6H5Cl | 112.56 | 440 | 0.74 | .007 | 0.6 | 342 | 1.6 | |||||
| p | 0.79 | 0.7 | 0.715 | ||||||||||||
| b-6 | benzil | n | C18H15N | 210.23 | 247 | 0.92 | 223 | 150 | 0.0051 | ||||||
| p | 2.0 | 227 | 0.67 | 1500 | 0.0056 | ||||||||||
| b-7 | benzoic acid | n | C7H6O2 | 112.12 | 0.0068 | 324 | 3.06 | ||||||||
| p | 428 | 326 | 0.27 | 2.1 | |||||||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| b-8 | benzonitrile | n | C7H5N | 103.04 | 320 | ||||||||||
| p | 0.30 | 323 | 0.43 | 3.10 | |||||||||||
| b-9 | benzophenone | n | C13H10O | 182.22 | 316 | 0.030 | 4x10-6 | 0 | 1.0 | 287 | 6.9 | ~0.0001 | |||
| p | 311 | 0.016 | 1 | 289 | 0.84 | 50 | 0.006 | ||||||||
| b-10 | benzo[a}pyrene | n | C20H12 | 252.31 | 295 | 49 | 175 | 8700 | |||||||
| p | 297 | 27.3 | 0.42 | 177 | 8800 | ||||||||||
| b-11 | benzo[e]pyrene | n | C20H12 | 252.31 | 325 | ||||||||||
| p | 327 | 221 | |||||||||||||
| b-12 | benzoquinone | n | C6H4O2 | 108.10 | 262 | 224 | |||||||||
| p | 0.53 | ||||||||||||||
| b-13 | biacetyl | n | C4H6O2 | 86.09 | 11.5 | 0.0027 | 1.0 | 638 | |||||||
| p | 267 | 7.7 | 236 | 0.23 | 145 | 0.00225 | |||||||||
| b-14 | biphenyl | n | C12H10 | 154.21 | 418 | 16.0 | 0.15 | 0.84 | 274 | 130 | |||||
| p | 391 | 274 | |||||||||||||
| b-15 | butadiene | n | C4H8 | 54.09 | 249 | ||||||||||
| p | |||||||||||||||
| b-16 | -1,4-diphenyl | n | C16H14 | 206.28 | 0.60 | 0.41 | 0.020 | 1.6 | |||||||
| p | 334 | 0.060 | 0.042 | <0.003 | 5.0 | ||||||||||
| c-1 | carbazole | n | C12H9N | 167.21 | 361 | 16.1 | 0.31 | 0.36 | 170 | ||||||
| p | 347 | 15.2 | 15 | 0.42 | 0.44 | 0.23 | 294 | 0.24 | 6.8 | ||||||
| c-2 | b-carotene | n | C40H56 | 536.87 | 228 | 0.0084 | <0.001 | 88 | 70 | ||||||
| p | 85 | 9 | |||||||||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| c-3 | chlorophyll a | n | C55H72MgN4O5 | 893.51 | 177 | 7.8 | 0.32 | 0.35 | 0.65 | 1500 | 0.002 | ||||
| p | 178 | 5.5 | 0.33 | 0.54 | 0.53 | 125 | 1x10-5 | 800 | 0.0014 | ||||||
| c-4 | chlorophyll b | n | C55H70MgN4O6 | 907.47 | 181 | 6.3 | 0.11 | 130 | 2500 | ||||||
| p | 179 | 3.5 | 0.12 | 0.14 | 0.81 | 136 | 3x10-5 | 1500 | 0.0028 | ||||||
| c-5 | chrysene | n | C18H12 | 228.29 | 331 | 44.7 | 0.12 | 0.85 | 710 | 2.20 | |||||
| p | 332 | 42.6 | 55 | 0.17 | 0.23 | 0.85 | 0.70 | 239 | 0.050 | 2.54 | |||||
| c-6 | coronene | n | C24H12 | 300.36 | 279 | 307 | 8.45 | ||||||||
| p | 279 | 320 | 320 | 0.23 | 0.27 | 0.56 | 0.68 | 228 | 0.12 | 9.5 | |||||
| c-7 | 1,3-cyclohexadiene | n | C6H8 | 80.13 | 410 | 223 | 30 | ||||||||
| p | |||||||||||||||
| d-1 | dibenz[a,h]anthracene | n | C22H14 | 278.35 | 303 | 37 | 0.90 | ||||||||
| p | 303 | 27 | 45.5 | 0.12 | 0.19 | 0.9 | 0.98 | 218 | 0.021 | 1.60 | |||||
| d-2 | diphenylamine | n | C12H11N | 169.23 | 0.05 | 0.32 | 0.3 | ||||||||
| p | 372 | 2.4 | 2.6 | 0.11 | 0.12 | 0.47 | 0.87 | 301 | 0.74 | 0.5 | 1.2 | ||||
| e-1 | eosin dianion | n | C20H6Br4Na2O5 | 647.89 | |||||||||||
| p | 209 | 3.6 | 0.69 | 0.33 | 0.022 | 177 | 0.022 | 1700 | 0.0093 | ||||||
| f-1 | fluoranthene | n | C16H10 | 202.26 | 295 | 53 | 0.35 | 221 | 0.84 | ||||||
| p | 295 | 58 | 0.21 | 221 | 8500 | 0.99 | |||||||||
| f-2 | fluorene | n | C13H10 | 166.23 | 397 | 10 | 0.68 | 0.83 | 0.22 | 282 | 150 | 5.1 | |||
| p | 397 | 0.68 | 0.8 | 0.32 | 284 | 0.07 | 5.7 | ||||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| f-3 | fluorenone | n | C13H8O | 180.92 | 2.8 | 0.94 | 500 | ||||||||
| p | 266 | 21.5 | 20 | 0.09 | 0.48 | 211 | 3x10-5 | 100 | 0.0019 | ||||||
| f-4 | fluorescein dianion | n | C20H12O5 | 332.31 | |||||||||||
| p | 230 | 3.6 | 0.97 | 0.97 | 0.02 | 00.78 | 197 | 0.0003 | 20000 | 0.3 | |||||
| i-1 | 1-indanone | n | C9H8O | 132.16 | 331 | <10-6 | 314 | ||||||||
| p | 317 | ||||||||||||||
| i-2 | indole | n | C8H7N | 117.15 | 415 | 7.9 | 0.33 | 0.43 | 0.34 | 301 | 16 | ||||
| p | 401 | 4.6 | 0.42 | 0.6 | 0.23 | 296 | 11.6 | 2.4 | |||||||
| i-3 | isoprene | n | C5H8 | 68.12 | 251 | ||||||||||
| p | |||||||||||||||
| n-1 | naphthalene | n | C10H8 | 128.17 | 385 | 96 | 262 | 0.19 | 0.45 | 0.75 | 253 | 0.033 | 175 | 2.3 | |
| p | 384 | 105 | 273 | 0.21 | 0.45 | 0.80 | 0.29 | 255 | 0.039 | 1800 | 2.6 | ||||
| n-2 | -d8 | n | C10D8 | 136.22 | 382 | 96 | 304 | 0.27 | 0.50 | >0.38 | 0.16 | 18.5 | |||
| p | 250 | 0.22 | 0.41 | 0.25 | 0.34 | 20.4 | |||||||||
| n-3 | -1-acetyl | n | C12H10O | 170.21 | |||||||||||
| p | 249 | 300 | |||||||||||||
| n-4 | - 2-acetyl | n | C12H10O | 170.21 | 0.84 | 249 | |||||||||
| p | 325 | ||||||||||||||
| n-5 | - 1-amino | n | C10H9N | 143.19 | 348 | 6.0 | 10.9 | 0.47 | >0.15 | ||||||
| p | 324 | 19.6 | 15.1 | 0.57 | 229 | 1.5 | |||||||||
| n-6 | - 2-amino | n | C10H9N | 143.19 | 6.9 | 20.6 | 0.33 | 0.80 | 0.58 | ||||||
| p | 306 | 16.6 | 20.5 | 0.46 | 0.32 | 239 | 0.0024 | 1.2 | |||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| n-7 | - 1,4-dicyano | n | C12H8N2 | 178.19 | 356 | 3.4 | |||||||||
| p | 10.1 | 0.19 | 232 | 40 | |||||||||||
| n-8 | -1-methoxy | n | C11H10O | 158.20 | 0.36 | 0.45 | |||||||||
| p | 374 | 0.53 | 0.50 | 250 | 5500 | ||||||||||
| n-9 | - 1-methyl | n | C11H10 | 142.20 | 377 | 67 | 0.21 | 0.58 | |||||||
| p | 377 | 97 | 0.19 | 254 | |||||||||||
| n-10 | - 2-methyl | n | C11H10 | 142.20 | 376 | 59 | 0.27 | 0.56 | |||||||
| p | 374 | 47 | 0.16 | 254 | |||||||||||
| n-11 | 2-naphthol | n | C10H8O | 144.17 | 362 | 13.3 | 0.27 | 67 | |||||||
| p | 362 | 8.9 | 252 | ||||||||||||
| o-1 | oxygen | n | O2 | 31.999 | 95 158 singlets |
||||||||||
| p | |||||||||||||||
| p-1 | cis-1,3-pentadiene | n | C5H8 | 68.12 | |||||||||||
| p | |||||||||||||||
| p-2 | trans-1,3-pentadiene | n | C5H8 | 68.12 | |||||||||||
| p-3 | perylene | n | C20H12 | 252.32 | 275 | 6.4 | 0.75 | 0.014 | 148 | ||||||
| 273 | 6.0 | 0.87 | 0.0088 | 151 | 5000 | ||||||||||
| p-4 | phenanthrene | n | C14H10 | 178.23 | 346 | 57.5 | 0.14 | 0.73 | 260 | 145 | |||||
| 345 | 60.7 | 63 | 0.13 | 0.20 | 0.85 | 0.46 | 257 | 0.16 | 910 | 3.6 | |||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| p-5 | phenazine | n | C12H8N2 | 180.21 | 273 | 0.014 | 0.21 | 186 | 42 | ||||||
| p | 299 | 0.020 | 0.0015 | 0.45 | 187 | 770 | |||||||||
| p-6 | phenol | n | C6H6O | 94.11 | 431 | 2.1 | 0.066 | 0.32 | |||||||
| p | 423 | 7.0 | 0.19 | 0.29 | 342 | 3.3 | |||||||||
| p-7 | phthalazine | n | C8H6N2 | 130.15 | 0.19 | 0.44 | 264 | 2.7 | |||||||
| p | 309 | 0 | 0.49 | 275 | 21.3 | 0.42 | |||||||||
| p-8 | porphine, tetraphenyl- | n | C44H30N4 | 614.72 | 179 | 13.6 | 0.11 | 0.82 | 138 | 1500 | |||||
| p | 185 | 10.1 | 0.15 | 0.88 | 140 | ||||||||||
| p-9 | pyrazine | n | C4H4N2 | 80.09 | 365 | 0.0004 | 0.0006 | 0.33 | 315 | 0.30 | 0.0185 | ||||
| p | 515 | 0.87 | 311 | 4.5 | 0.020 | ||||||||||
| p-10 | pyrene | n | C16H10 | 202.25 | 322 | 650 | 0.65 | 0.88 | 0.37 | 203 | 0.0021 | 180 | 0.55 | ||
| p | 321 | 190 | 0.72 | 0.92 | 0.38 | 0.22 | 202 | 0.022 | 11000 | 0.58 | |||||
| p-11 | pyridine | n | C5H5N | 79.10 | 415 | 0.005 | 0.004 | 0.7 | |||||||
| p | 0.025 | 0.015 | 2.4 | ||||||||||||
| p-12 | pyrimidine | n | C4H4N2 | 80.09 | 360 | 1.5 | 0.0029 | 00.58 | 0.12 | 338 | 0.14 | ||||
| p | 1.0 | 1.4 | |||||||||||||
| q-1 | quinoline | n | C9H7N | 129.16 | 383 | <10-5 | 0.31 | 0.43 | 258 | 1.04 | |||||
| p | 381 | 261 | |||||||||||||
| r-1 | rhodamine B | n | C28H31ClN2O3 | 479.02 | |||||||||||
| p | 213 | 2.7 | 0.65 | 1.0 | 0.0024 | 0.003 | 178 | 5x10-5 | 1.6 | ||||||
| r-2 | (all-E)-retinol | n | C20H30O | 286.45 | 321 | 5.0 | 0.02 | 0.017 | 140 | 25 | |||||
| p | |||||||||||||||
| s-1 | (E)-stilbene | n | C14H12 | 180.25 | 358 | 0.075 | 0.036 | 206 | 14 | 0.1 | |||||
| p | 0.016 | 0.90 | 206 | 62 | 0.022 | ||||||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl (77K) |
fisc |
fisc (77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec 77K) |
| s-2 | styrene | n | C8H8 | 104.15 | 415 | 13.9 | 18.8 | 0.25 | 0.46 | 0.40 | 258 | <0.001 | 0.025 | ||
| p | |||||||||||||||
| t-1 | p-terphenyl | n | C18H14 | 230.31 | 385 | 0.95 | 0.77 | 0.11 | 450 | ||||||
| p | 1.3 | 244 | 2.6 | ||||||||||||
| t-2 | tetracene | n | C18H12 | 228.29 | 254 | 6.4 | 0.17 | 0.62 | 123 | 400 | |||||
| (napthacene) | p | 254 | 0.16 | 0.66 | |||||||||||
| t-3 | toluene | n | C7H8 | 92.14 | 445 | 34 | 53.3 | 0.14 | 0.29 | 0.53 | 346 | 7.73 | |||
| p | 35 | 0.13 | 0.27 | 347 | 7.7 | ||||||||||
| t-4 | triphenylene | n | C18H12 | 228.29 | 349 | 36.6 | 0.066 | 0.86 | 55 | ||||||
| p | 0.07 | 0.54 | 0.41 | 15.2 | |||||||||||
| x-1 | xanthone | n | C13H8O2 | 196.19 | 324 | 0.013 | 310 | 0.02 | |||||||
| p | 0.008 | 310 | 17.9 | ||||||||||||
| x-2 | o-xylene | n | C8H10 | 106.16 | 438 | 32.2 | 62.3 | 0.18 | 0.33 | 0.28 | 343 | 0.45 | 7.46 | ||
| p | 38 | 344 | 5.2 | ||||||||||||
| x-3 | m-xylene | n | C8H10 | 106.16 | 439 | 30.8 | 0.14 | 0.28 | 339 | 0.25 | |||||
| p | 336 | 8.1 | |||||||||||||
| x-4 | p-xylene | n | C8H10 | 106.16 | 435 | 30 | 0.22 | 0.45 | 0.63 | 337 | 0.49 | 4.6 | |||
| p | 33.7 | 0.24 | 337 | 6.2 | |||||||||||
| # | Name | n = nonpolar p= polar |
Formula | Molecular
Mass (g/mol) |
Es
(KJ/mol) |
ts
(nsec) |
tfl
(nsec 77K) |
ffl |
ffl
(77K) |
fisc |
fisc
(77K) |
Et
(KJ/mol) |
fph
(77k) |
tt
(msec 20oC) |
tt
(sec) |
|
cmpd. # |
cmpd. name | solvent polarity | formula | molecular mass | lowest excited singlet energy | singlet lifetime room temp. | fluor. lifetime 77K | fluor. quantum yield room temp. | fluor. quantum yield 77K | triplet quant. yield room teimp. | triplet quantum yield 77K. | lowest triplet energy | phos. quantum yield 77K | triplet lifetime room temp. | triplet lifetime 77K |
The values in this table have been selected according to the bias of the author (admittedly out of touch with the field of photochemistry) and from the number of entries for the compounds in the index of Murov, S. L.; Carmichael, I.; Hug, G. L. Handbook of Photochemistry, 2nd ed., 1993, Marcel Dekker, N.Y. The data has been extracted from the aforementioned Handbook. A third edition of the Handbook publilshed in 2007 includes the addition of about 10% more compounds and some new tables. However, the 3rd edition, while containing a subject index does not contain the compound (name) index and formula index that are included in the 2nd edtion. Despite the fact that a significant portion of the 3rd edition was taken from the 2nd edition, the authors of the 2nd edition were not cited on the cover of the 3rd edition.
Use of the above table as a primary reference is strongly discouraged and even reference to one of the handbooks is not the best practice as the original sources should be cited. This table was posted primarily as a quick and handy source for data that could be useful for experimental design and references are not included. To find references for the sources data, please refer to one of the Handbooks and then to the original source especially if the data is to be cited. In addtion to the 2nd edition of the Handbook, the thesis of S. Murov at the University of Chicago (!967) entitled the Photochemistry of Aromatic Ketones included some compounds not published in the handbooks. Some of the data from the thesis has been included in and addendum to this site (please visit http://murov.info/murovthesis.pdf ).
If you have find errors or would like to suggest other compoounds for inclusion in the table, your comments will be deeply appreciated. Steven Murov, murovs@yosemite.edu http://murov.info http://murov.info/resume.htm
hit counter