CHEMISTRY DEMONSTRATIONS (#2):
Hints for Presentation Techniques, Steven Murov,
Professor Emeritus of Chemstry, Modesto
Junior College
murovs@yosemite.edu
last modified
8/7/21,
some link checks on 1/29/21
On 8/15/21, a new demo web site has been posted
that contains similar demos but organized into themes intended for
approximately 20 minute presentations.
http://murov.info/chemdemos3.htm
This web site was split on 1/30/21 into two
websites, this page (http://murov.info/chemdemos2.htm) and the original site: http://murov.info/chemdemos.htm.
This site includes a different narrative and emphasizes the
American flag demo.
A PowerPoint program has been designed to be
used with the flag demo and is available at:
http://murov.info/NationalAnthem(U.S.).ppt
For many more websites
by this author, please visit: http://murov.info
Free
downloadable lab book for the college level organic chemistry course just
posted 02/27/14.
Experiments and Exercises in Organic Chemistry: A Challenge Oriented
Approach -
http://murov.info/orglab.htm
The
Internet contains a substantial number of sites related to
chemistry.
A number of
sites give information about chemical demonstrations. Some of these are
available in the parent site (http://murov.info/webercises.htm)
and in the list
below.
While the discussion presented below does include instructions for some
chemistry demonstrations, the intention of this
Internet
site is not to provide an extensive set of demonstrations but to provide some
ideas for presentation style and technique and also an anticipation of audience
response.
The following sites contain chemical demonstrations:
http://murov.info/chemdemos.htm
 http://www.thecatalyst.org/m05demos.html
http://www.thecatalyst.org/m05demos.html
http://www.elmhurst.edu/~chm/demos/
http://www.chymist.com/demonstrations.html
http://www.interactives.co.uk/hearts_dinner.htm
http://www.chemmybear.com/hallownotes.html
https://sites.google.com/site/acsteacheraffiliates/the-demo-den
http://www.practicalchemistry.org
http://www2.chem.uic.edu/marek/clips/marek_chem_demos.htm
http://resources.schoolscience.co.uk/Salters/chemclub2_9.html
http://graysci.com/main-index/
http://chemistry.about.com/od/demonstrationsexperiments/
http://www.sciencebob.com/index.php
http://chemistry.about.com/od/demonstrationsexperiments/tp/myfavorites.htm
http://www.rsc.org/eic/exhibition-chemistry
https://edu.rsc.org/resources/collections/classic-chemistry-demonstrations
https://pubs.acs.org/doi/suppl/10.1021/acs.jchemed.7b00835/suppl_file/ed7b00835_si_001.pdf
https://www.unco.edu/nhs/science/pdf/demos/Chemistry_Demos.pdf
http://www.scifun.org/
https://www.thoughtco.com/cool-chemistry-demonstrations-604264
https://www.stem.org.uk/resources/collection/4031/classic-chemistry-demonstrations
https://www.chem.indiana.edu/faculty-research/faculty-resources/chemistry-demos/
https://chem.washington.edu/lecture-demos
Videos
https://www.youtube.com/playlist?list=PL1Oi4O0iZ7iZshV0w2Jxx0XQRqH5ro3DZ
http://www.scifun.org/ScienceVideos/videos.htm
http://www.kentchemistry.com/KentsDemos.htm
https://chemagic.org/demovideos/Index.html
https://netsacs.org/2020/07/11/enjoy-the-chemistry-demonstration-videos-from-science-elites/
https://www.youtube.com/watch?v=bOuEJf8Dr_4
https://chem.washington.edu/lecture-demos
https://science.psu.edu/chem/undergrad/demonstrations
https://www.youtube.com/watch?v=bOuEJf8Dr_4
https://www.stevespanglerscience.com/lab/categories/experiments/chemistry/
Safety in Chemical Demonstrations
https://www.acs.org/content/acs/en/chemical-safety.html
https://www.acs.org/content/acs/en/chemical-safety/teach-and-learn/safer-demonstrations.html
https://www.acs.org/content/acs/en/about/governance/committees/chemical-safety/publications-resources.html
https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/2015-2016/december-2015/safety-data-sheets.html
https://www.acs.org/content/acs/en/chemical-safety/resources.html
http://www.chymist.com/Safety with chemical
demonstrations.html
https://www.vforteachers.com/essay_5_ECS.htm
https://blog.cinfin.com/2015/12/15/science-labs/
https://www.sciencebuddies.org/science-fair-projects/references/chemistry-safety
https://www.cdc.gov/niosh/docs/2007-107/pdfs/2007-107.pdf
https://teachchemistry.org/classroom-resources/safety
https://www.snc.edu/chemicalhygiene/docs/labsafety/Safety_Guidelines_for_Chemical_Demonstrations.pdf
Audience
Participation Chemistry Demonstrations Including the American
Flag Demo
Abstract:
Chemistry
demonstrations that illustrate the importance and excitement of science should
stimulate interest in science and have a positive impact on the science
literacy of the audiences. To increase audience attention and learning and to
dispel the aura of “magic” that sometimes accompanies
chemistry presentations, it is beneficial to involve the audience in
interactive ways during the show. Methods of including audience participation
are discussed for several demonstrations with the emphasis on the preparation
of an American flag while the audience sings the National
Anthem.
Audience Participation Chemistry Demonstrations Including the American Flag Demo
Chemistry presentations for K-12 students
and general audiences that illustrate the importanceand excitement of
science should stimulate interest in science and have a positive impact on the
scienceliteracy of the audiences (1-15). To increase audience attention and
learning and to dispel the aura of“magic” that sometimes accompanies
chemistry presentations, it is beneficial to involve the audience
in interactive ways during the show. Methods of including audience
participation are discussed for several demonstrations with the emphasis on
the preparation of an American flag while the audience sings the National
Anthem.
http://murov.info/NationalAnthem(U.S.).ppt
Instructions
follow in the supplement below.
Public events often begin with the singing of the National Anthem. This
tradition is appropriate for the opening of a chemistry show. The words to
the National Anthem are projected using a computer or an overhead
transparency. Another option is to use or develop a PowerPoint presentation
that illustrates the Anthem line by line (16). Shortly before the beginning
of the show, a few people are selected from the audience and each is given a
rocket balloon and an air pump. The audience is asked to stand and at the
count of 3 to sing the Anthem. As the audience starts singing, the “rocket
people” should fully inflate their balloons and hold onto them. When the
singing reaches rockets red glare, the balloon holders should release the
balloons aimed up and toward the back of the audience. When the singing
reaches bombs bursting, the presenter should explode a carbide cannon (17) or
another safe type of explosive. Finally, when the singing reaches the flag
was still there, the presenter should spray aqueous FeCl3 on a
flag previously prepared with aqueous KSCN stripes and aqueous
K4Fe(CN)6 background for the stars (18-20). Details for
preparation of the flag and possible alternative chemicals such as the use of
acid base indicators are included in the supplementary online material. The
flag demonstration can be used as a teaching moment to emphasize the importance
of observation in science. After singing the Anthem, the audience can be
asked if the Anthem has ever led to the questioning of any of the contents of
the Anthem such as the meaning of the word ramparts. Despite the fact that
the audience has heard or sung the Anthem dozens of times, most people
have overlooked the fact that they don’t know the meaning of ramparts and
could want to improve their observational skills. A picture that illustrates
the National Anthem and contains ramparts is available online (21). It is
interesting to ask and discuss what was meant by rockets red glare when the song
was published in 1814. The audience can also be asked how many are aware that
there are several more verses to the Anthem. At the risk of starting a
political debate, the audience can be asked if it is appropriate for the
Anthem to focus on war instead of peace or the beauty of our country (e.g., see
the 03/02/09 Get Fuzzy comic strip by Darby Conley). Finally, some humor can
be added by informing the audience that a critique of their singing is in
vogue. This can be followed by showing a picture of American Idol’s Simon
Cowell (22).
Other demonstrations that are nicely suited for audience participation
include the clock, oscillating clock, a modified blue bottle, nylon, sodium
polyacrylate, vacuum and pressure and liquid nitrogen experiments. For the
clock reaction, it has been suggested that many solutions be prepared
to change color at climactic moments in the William Tell Overture (another
option is the 1812 Overture) (23). The preparation for this is time consuming
and the demo is difficult to time properly. A simple alternative is to tell
the audience to count slowly, loudly and in unison when two solutions are
mixed. Before mixing, predict the number on which the change will occur but
point out there is a considerable margin of error. For the oscillating clock,
in advance, tell the audience to lean collectively to the right and say ooh
when the solution turns dark blue. When the solution turns colorless, they
should lean to the left and say aah. They can relax and sit straight up when
it is briefly yellow. Be sure to let the audience know when they can stop
reacting to the demonstration.
A
valuable lesson can be taught with the use a modified blue bottle experiment
(contains methylene blue, resaurzin, sodium hydroxide and glucose) (24). This
reaction should be started early in the program with the alert that the
audience should glance at it occasionally. After it turns colorless (except
for an important tinge of color on the top surface that the audience will not be
able to observe), one swirl should turn it red and vigorous stirring turns it
blue. The standing, stirring cycles can be repeated periodically until you
are ready to discuss the reaction. Most audiences when asked why the red and
blue appear with mixing usually respond that the stirring adds energy or that
the color at the top is just being mixed around. Usually it takes the hint
that a substance is being added as a result of stirring to lead people to
suggest that the color change is due to air. It is now appropriate to ask what
the three most abundant gases in dry air are. Few people seem to know that
nitrogen is number one and it is very rare that anyone knows that argon is
number 3. If you want to introduce a pun, write ABCDEFGHIJKLMNOPQSTUVWXYZ on
a board and tell the audience that the letters represent the third most
abundant gas in dry air. If someone responds “the alphabet”, remind the audience
of the importance of complete, careful and unbiased observations. Someone
will usually then say that the R is missing which then slowly but surely
leads to the recognition that argon is the third most abundant gas. Even more
important, usually someone will say that carbon dioxide is one of the top three
and some people think it is number one. This is another great teaching moment
as you can point out that carbon dioxide is number 4 but currently makes up
only 0.038% of the atmosphere. Because there is not much present, human
activities have caused a 36% increase in the carbon dioxide content and it will
continue to increase and arguably stress global climates unless society
quickly find alternates to fossil fuel consumption.
Nylon synthesis and the use of sodium polyacrylate (diaper powder) are
good ways to lead in to a discussion of the importance of chemistry.
Elementary school students when asked if they use human-made chemicals usually only name cosmetic products. The
students have just never thought about or been asked to think about the
source of products such as medicines, plastics and synthetic fabrics. Many
other demonstrations can be used to initiate meaningful discussions with an
audience. Vacuum demonstrations including expanding marshmallows and balloons
and collapsing containers (when evacuated) can be used to discuss pressure
and the nature of sound and light waves. If liquid nitrogen is available,
tell the audience you are going to do several experiments with a
mysterious substance and ask the audience to be good observers of the
experiments. Tell them you will ask them several questions at the end of the
demonstrations:
a. What color is
the substance? Sometime during the presentation, ask the audience the color
of water and point out why clear, transparent or white are either not answers
to the question or incorrect. This does raise the question whether the
terminology “white wine” is appropriate.
b. Is it hot, room temperature or cold?
c. Is it a solid, liquid or gas?
d. What is the substance?
Once the
attention of the audience has been attained, the opportunity to discuss the
importance of science literacy should not be passed up. Ask the audience how
they would vote on possible construction of a nuclear fusion energy facility
in their community. Most people react to the word nuclear and do not know the
difference between fusion and fission. Show pictures drawn by students that
depict scientists as mad (almost all pictures show a lone crazy looking white
male scientist with either no hair or very strange hair) (25-29). Use the
nylon, liquid nitrogen and other demonstrations to point out the importance
of science and discuss the very misguided stereotype of a scientist. A
scientifically literate society is needed if we are going to make the best
decisions in the future and you will be rewarded with the audience reaction
to your presentation.
1. Louters, L. L.; Huisman, R. D.,
J. Chem.
Ed.,1999,
76, 196.
2. O'Brien, T., J. Chem. Ed.,
1991,
68, 933.
3. Sullivan, D. M.,
J. Chem. Ed.,
1990,
67, 887.
4. Fenster, A. E.; Harpp, D. N.; Schwarcz, J.
A., J. Chem. Ed.,
1985,
62, 1100.
5. Waterman, E. L.;
Bilsing, L. M., J. Chem. Ed.,
1983,
60, 415.
6.
Bergmeier, B. D.; Saunders, S. R., J. Chem. Ed.,
1982,
59,
529.
7. Katz, D.,
J. Chem. Ed.,
1991,
68, 235.
8.
Wolfe, R., J. Chem. Ed.,
1990,
67, 1008.
9. Stamm, D.
M.; Franz, D. A., J. Chem. Ed.,
1992,
69, 762.
10. Ihde,
J., J. Chem. Ed.,
1990,
67, 264.
11. Wright, S. W.;
Cotton, W. D.; Hess, V. G., J. Chem. Ed.,
2002,
79,
44.
12. McRae, R.; Rahn, J. A.; Beamer, T. W.; LeBret, N.,
J. Chem.
Ed., 2002,
79, 1220.
13. Meyer, L. S.; Schmidt, S.; Nozawa,
F.; Panee, D. J. Chem. Ed.,
2003,
80, 431.
14. Roadruck,
M. D., J. Chem. Ed.,
1993,
70, 1025.
15. Ophardt, C. E.;
Applebee, M. S.; Losey, E. N., J. Chem. Ed.,
2005,
82,
1174.
16. Murov, S.,
http://murov.info/NationalAnthem(U.S.).ppt
or an older version at http://www.slideshare.net/murovs/national-anthem-3064731
(accessed
08/22/10).
17. Conestoga Company’s Big Bang Cannon,
http://www.bigbangcannons.com/
.
18. Chen, P. S.,
Entertaining and Educational Chemical Demonstrations, Chemical Elements
Publishing
Co., 1974, 20.
19. Reising, J. M.; Nguyen, P. N.; Flint, E. B.;
Campbell, D. J., Chem. Educator,
2007,
12, 85-88.
20. Old Glory: A Patriotic
Colors Demonstration,
http://www.flinnsci.com/media/395499/cf0438.00.pdf
21. Battle for Fort
McHenry,
http://helpmejoseph.typepad.com/charlotte_front_and_cente/images/2008/07/04/4_mural_fort_mchenry.jpg
(accessed 03/05/09).
22. For example, see:
http://i1.cdnds.net/13/41/618x426/simon-cowell-1.jpg
23. Brice, L. K.,
J. Chem. Ed.,
1980,
57, 152
24. Chen, P. S.,
Entertaining and Educational Chemical Demonstrations, Chemical Elements
Publishing
Co., 1974, 38.
25. Sjoberg, S., Science And Scientists: The SAS-study,
http://folk.uio.no/sveinsj/SASweb.htm (accessed
03/02/09).
26. Google "Matkins
Drawing a Scientist."
27. Science and Technology:
Public Attitudes and Public Understanding,
http://www.nsf.gov/statistics/seind02/c7/c7s3.htm (accessed 03/02/09).
28. Nuno, J., Draw a Scientist:
Middle School and High School Students’ Conceptions about Scientists,
http://www.jdenuno.com/Resume%20Web/DAST.htm (accessed 03/02/09).
29. Murov, S., Pictures of
Alchemists by the David Teniers,
http://murov.info/alchemists.htm (accessed 03/05/09).
Audience
Participation Chemistry Demonstrations: Online
Supplement
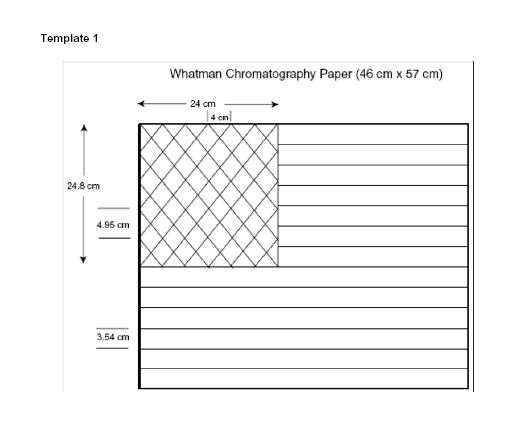
American flag.1 General findings included
the following: For paper, Whatman #1 Chromatography paper costs more than
other absorbent paper at about $2/sheet (46 cm x 57 cm sheet which can be cut
into four 8.5 inch x 11 inch sheets) but a less expensive alternative is
available from Sargent-Welch at
Chromatography Paper | Sargent Welch . Watercolor
and construction papers were easier to paint along lines but spraying usually
resulted in running of most of the colors. The red from 5% aqueous KSCN + 2%
aqueous FeCl3 spray ran the most but acid base indicators phenolphthalein
and thymolphthalein + 0.1 M NaOH spray also ran. Only the blue from 5%
aqueous K4Fe(CN)6 appeared to bind to the watercolor
and construction papers and not run. For large audiences, use of the whole 46 cm
x 57 cm sheet is desirable for adequate visibility but requires much more
preparation time than use of an 8.5 inch x 11 inch sheet (adequate for
audiences of 100 or less). The large sheet requires pencil lining by hand
using template 1 and substantial painting time while the 8.5 inch x 11 inch
sheet can be lined with a printer using the template below and painting time
is relatively short. Alternatives for colors include 5% aqueous KSCN for red
and 5% aqueous K4Fe(CN)6 for blue with spraying with 2%
aqueous FeCl3 or 0.5% phenolphthalein in 95% ethanol for “red”
(quotation marks because the red is reddish-pink) and 0.5% thymolphthalein in
95% ethanol for blue with spraying with 0.1 M NaOH. The acid-base
indicators have the disadvantage that 0.1 M NaOH is caustic and must be
sprayed very carefully away from any people. In addition, the blue lasts only
a few minutes before fading (presumably due to carbon dioxide in the air).
The fading could be used in conjunction with the blue bottle experiment to
discuss the issue of carbon dioxide and global climate change but has the
disadvantage that the fading of the blue contradicts the “flag was still
there” theme of the National Anthem. The K4Fe(CN)6 has the
disadvantage that yellow, tarts to appear about a week after painting so
painting should be performed within a few days of use.
For multiple copies of flags, use pencil on
a piece of Whatman 46 cm x 57 cm Chromatography paper or less expensive Sargent
Welch paper
https://www.sargentwelch.com/store/catalog/product.jsp?product_id=8869823 to draw the lines as
indicated in Template 1 to make a large flag. Technically, the paper does not
have the correct length to width ratio for an American flag2 but it is easier to use the paper as it comes from
the box. If you choose to cut it to the proper ratio of dimensions, then all
measurements will need to be recalculated. Use Template 1 to mark one flag
and use that marked flag as the template for all future flags. Tape the flag
to a piece of cardboard or mat board before applying the solutions. Painting
should be done at least a couple of hours before use to allow for drying. Use
about a 3/4 inch brush to paint the stripes with 5% aqueous potassium
thiocyanate solution (or 0.5% phenolphthalein in 95% ethanol). Because the
solution will spread about 1/8 inch in both vertical directions, some space
should be left between the application and the pencil lines. For the star
portion of the flag, use a small brush (about 1/4 nch) and paint a frame
with 5% aqueous potassium ferrocyanide (or 0.5% thymolphthalein in
95% ethanol) around the star section. Now paint diagonally in both directions
between the pencil lines leaving intersections of the pencil lines dry. There
also might be a few spots around the edges that need to be filled in with the
potassium ferrocyanide solution. Only one student out of hundreds has
ever complained that the stars that result are distorted circles rather than
stars. After the paper is dry, use a spray bottle to apply 2% aqueous
iron(III) chloride (or 0.1 M NaOH). The words to the National Anthem are
available online.
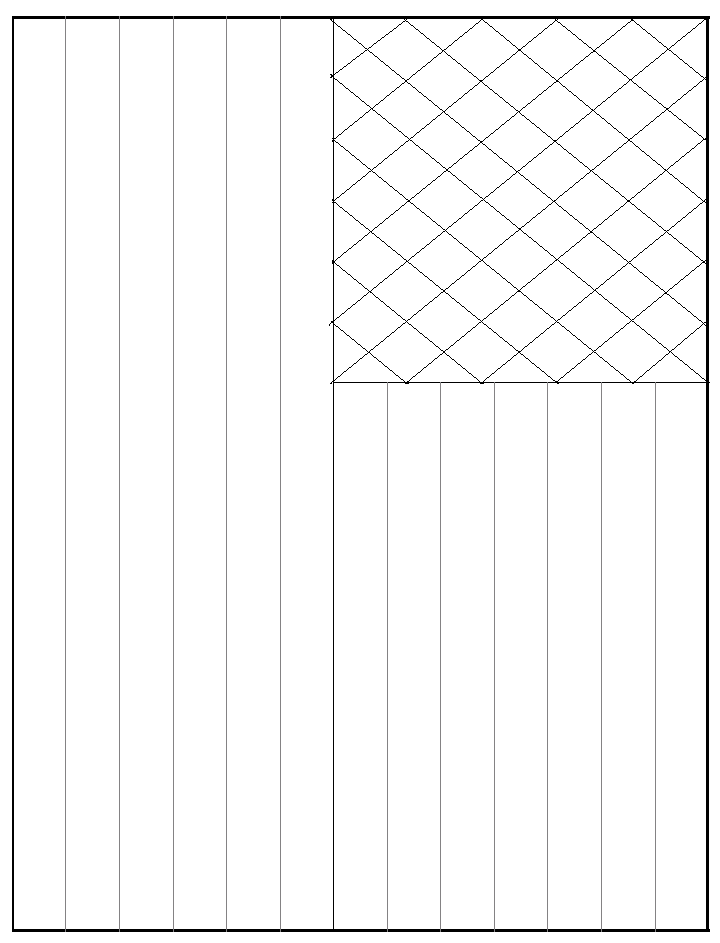
For smaller flags, cut the 46 cm x 57 cm sheet Whatman paper into 8.5
inch x 11 inch pieces and run them through a printer capable of dealing with
relatively thick paper using template 2 or 3. Paint as above. For the star
portion, paint around the region and then diagonally between the stars making
no attempt to make actual stars.3
Clock Reaction. Prepare the following
two solutions in plastic botles:
Solution A: Dissolve 4 g of soluble starch
in 1 L of boiling water. After cooling, add 2 g of
Na2S2O5.
Solution B: Dissolve 2 g
KIO3 in 1 L of water containing 0.3 mL of concentrated sulfuric
acid.
Mix: 25 mL of solution A and 25 mL of solution B (about 13 seconds for
color change)
Oscillating Clock
Reaction. Prepare the following solutions in plastic
bottles:
Solution A: Dilute 206 mL of 30% H2O2 to 500 mL with water. [Note:
27% hydrogen peroxide can be purchased under the trade name Baquacil Shock
and Oxidizer from pool stores. Check with http://www.baquacil.com/
for location of stores.]
Solution B: Dissolve 21.4 g of
KIO3 in 500 mL of water containing 2.2 mL of 18 M
H2SO4.
Solution C: Dissolve 3 g of soluble starch in
750 mL of boiling water. To the cooled solution, add 11.7 g of malonic acid
and 2.5 g of MnSO4
.
H2O.
Mix: Equal volumes (for small audiences, 25 mL of each is
sufficient) of solutions A, B, C in an Erlenmeyer flask. While the
demonstration is dramatic in an Erlenmeyer flask, it is even more
impressive if the solution is quickly transferred after mixing to a graduated
cylinder as there is a spatial effect to the oscillations that is easily
observable.
Blue Bottle
Experiment. The following four solutions are prepared
and stored in plastic bottles and dropper bottles.
Solution A: 32 g
KOH/500 mL water.
Solution B: 40 g dextrose/500 mL water.
Solution C: 0.04
g methylene blue/100 mL water.
Solution D: 1 g resaurzin (tablet form)/100 mL
water.
Mix about 30 mL of solution A, 30 mL of solution B, 10 drops of
solution C and 10 drops of solution D. Stir, allow to sit and turn pink and
then almost colorless (several minutes the first time and shorter amounts of
time later depending on the amount of stirring). Pick up the flask very
carefully and give it one quick swirl to turn it pink. Vigorous stirring will
turn the solution purple. The cycle can be repeated many
times.
Nylon. In a glass
bottle, prepare a 0.25 M adipyl chloride solution in cyclohexane. Transfer about
20 mL of this solution to a plastic dropper bottle. In a plastic bottle,
prepare an aqueous solution containing 0.5 M 1,6-diaminohexane and 0.5 M
NaOH. Put about 2 mL of the amine solution in a shallow dish or watch of copper wire with a small loop at its
end.
Sodium polyacrylate.
Add water to translucent container that has sodium polyacrylate in it. Turn
bottle upside down and nothing comes out. Add salt and stir and the aqueous
phase can be poured again. Mention the role of the powder in disposable
diapers to make the discussion relevant. An alternative is to play the 3 cup
trick with the audience. Put a small amount of sodium polyacrylate in a cup
before the audience enters the room.
Vacuum and pressure. Evacuate a bell
jar with marshmallows (or Peeps) inside. After expansion has maximized, a
small amount of contraction will take place. Then let air quickly reenter the
jar. Also suspend a tied off balloon with a small amount of air in the bell
jar. If it pops, the there will not be much sound and you can talk about the
transmission of sound waves. Attach the vacuum pump via a hose connected to
the appropriate size single hole rubber stopper to a large plastic bottle and
afterwards to a 5 gallon metal can (empty acetone cans that have been
thoroughly rinsed with water usually work). Generally, collapse occurs in a
very dramatic fashion.
Liquid
Nitrogen.
1. A balloon is attached to a piece of
vacuum hose with a hose clamp. The other end of the hose is connected to a
plastic bottle containing a small amount of liquid nitrogen.
2. An inflated
sealed balloon is slowly pushed into liquid nitrogen. Balloons that have bulges
or twists are more interesting. After the balloon has shrunk to a minimal
size, it is allowed to warm to room temperature. For college audiences, ask
why the volume decreases to close to zero rather than about 1/4 of the volume
as predicted approximately by Charles’ Law.
3. After liquid nitrogen is added
to a plastic bottle, a rubber stopper is securely inserted into the
bottle. After several seconds it will shoot out into the audience if aimed
properly.
4. Into the plastic bottle containing liquid nitrogen, insert a
rubber stopper with a 25 cm long piece of 7 mm tubing that reaches to near
the bottom of the bottle. A fountain will result and if sufficient nitrogen
is used, it is possible to almost disappear in the descending fog. The rubber
stopper should be held carefully as on rare occasions, liquid nitrogen runs
down the glass tube and can freeze small portions of your fingers.
5. A
marshmallow is placed on a copper wire and inserted into liquid nitrogen. After
about 3 minutes, gentle tapping in a beaker will shatter the
marshmallow.
6. Liquid nitrogen can be harmlessly poured quickly on the back
of your hand for very short periods of time.
7. Have the children separate
if the floor is carpeted and leave a wide aisle and throw some
liquid nitrogen onto the floor between them.
Templates 2 and 3 for 8.5 inch x 11
inch
chromatography paper are on next pages.
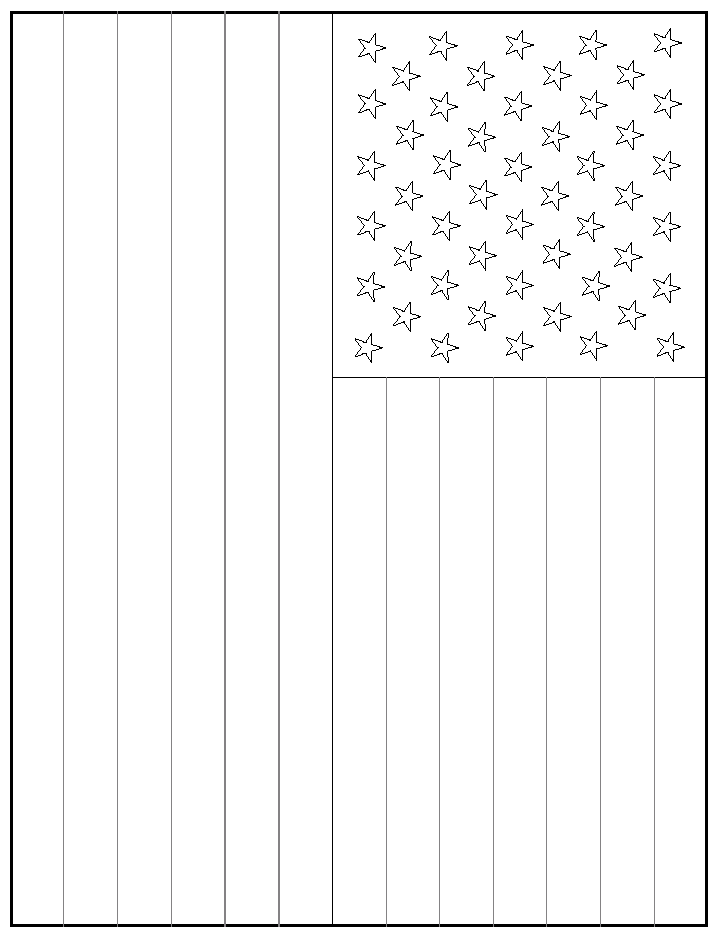
1. Chen, P. S., Entertaining and Educational Chemical
Demonstrations, Chemical Elements Publishing
Co., 1974, 20.
2.
American Flag Proportions, http://www.montney.com/flag/proportions.htm (accessed 03/05/09).
3. For example, see: The
National Anthem: The Star-Spangled Banner,
http://murov.info/NationalAnthem(U.S.).ppt
http://www.usflag.org/thenationalanthem.html (accessed 03/06/09), The Star Spangled Banner
http://www.scoutsongs.com/lyrics/starspangledbanner.html (accessed 03/06/09), Star Spangled Banner.
visitor number (restarted at zero 07/26/12),
updated 1/30/21
Web Counter
unique visitor hit counter -
started 1/30/21

Return to
top:
Jump to "Chemistry
Webercises Directory"
 http://www.thecatalyst.org/m05demos.html
http://www.thecatalyst.org/m05demos.html 

