c
CHEMISTRY DEMONSTRATIONS (#3):
Demonstrations
for Six* 20 minute themed presentations
*Two
bonus sections have been added.
Steven Murov,
Professor Emeritus of Chemstry, Modesto
Junior College
murovs@yosemite.edu
This web site
contains chemistry demonstrations derived from two previously posted web
sites. (http://murov.info/chemdemos.htm
and http://murov.info/chemdemos2.htm).
However, in this site they are arranged into six themes.
For many more websites
by this author, please visit: http://murov.info
Free
downloadable lab book for the college level organic chemistry course, visit:
Experiments and Exercises in Organic Chemistry: A Challenge Oriented
Approach -
http://murov.info/orglab.htm
The
Internet contains a substantial number of sites related to
chemistry.
A number of
sites give information about chemical demonstrations. Some of these are
available in the site (http://murov.info/webercises.htm)
and in the list
below.
While the discussion presented below does include instructions for some
chemistry demonstrations, the intention of this
Internet
site is not just to provide a set of demonstrations, but to provide some
ideas for presentation style and techniques and also an anticipation of audience
response.
The following sites contain
instructions for chemical demonstrations:
http://murov.info/chemdemos.htm
 http://www.thecatalyst.org/m05demos.html
http://www.thecatalyst.org/m05demos.html
http://www.elmhurst.edu/~chm/demos/
http://www.chymist.com/demonstrations.html
http://www.interactives.co.uk/hearts_dinner.htm
http://www.chemmybear.com/hallownotes.html
https://sites.google.com/site/acsteacheraffiliates/the-demo-den
http://www.practicalchemistry.org
http://www2.chem.uic.edu/marek/clips/marek_chem_demos.htm
http://resources.schoolscience.co.uk/Salters/chemclub2_9.html
http://graysci.com/main-index/
http://chemistry.about.com/od/demonstrationsexperiments/
http://www.sciencebob.com/index.php
http://chemistry.about.com/od/demonstrationsexperiments/tp/myfavorites.htm
http://www.rsc.org/eic/exhibition-chemistry
https://edu.rsc.org/resources/collections/classic-chemistry-demonstrations
https://pubs.acs.org/doi/suppl/10.1021/acs.jchemed.7b00835/suppl_file/ed7b00835_si_001.pdf
https://www.unco.edu/nhs/science/pdf/demos/Chemistry_Demos.pdf
http://www.scifun.org/
https://www.thoughtco.com/cool-chemistry-demonstrations-604264
https://www.stem.org.uk/resources/collection/4031/classic-chemistry-demonstrations
https://www.chem.indiana.edu/faculty-research/faculty-resources/chemistry-demos/
https://chem.washington.edu/lecture-demos
Videos
https://www.youtube.com/playlist?list=PL1Oi4O0iZ7iZshV0w2Jxx0XQRqH5ro3DZ
http://www.scifun.org/ScienceVideos/videos.htm
http://www.kentchemistry.com/KentsDemos.htm
https://chemagic.org/demovideos/Index.html
https://netsacs.org/2020/07/11/enjoy-the-chemistry-demonstration-videos-from-science-elites/
https://www.youtube.com/watch?v=bOuEJf8Dr_4
https://chem.washington.edu/lecture-demos
https://science.psu.edu/chem/undergrad/demonstrations
https://www.youtube.com/watch?v=bOuEJf8Dr_4
https://www.stevespanglerscience.com/lab/categories/experiments/chemistry/
Safety in Chemical Demonstrations
https://www.acs.org/content/acs/en/chemical-safety.html
https://www.acs.org/content/acs/en/chemical-safety/teach-and-learn/safer-demonstrations.html
https://www.acs.org/content/acs/en/about/governance/committees/chemical-safety/publications-resources.html
https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/2015-2016/december-2015/safety-data-sheets.html
https://www.acs.org/content/acs/en/chemical-safety/resources.html
http://www.chymist.com/Safety with chemical
demonstrations.html
https://www.vforteachers.com/essay_5_ECS.htm
https://blog.cinfin.com/2015/12/15/science-labs/
https://www.sciencebuddies.org/science-fair-projects/references/chemistry-safety
https://www.cdc.gov/niosh/docs/2007-107/pdfs/2007-107.pdf
https://teachchemistry.org/classroom-resources/safety
https://www.snc.edu/chemicalhygiene/docs/labsafety/Safety_Guidelines_for_Chemical_Demonstrations.pdf
Themed Chemistry Demonstrations.
1. Over the
Rainbow: The Colors of Chemicals
Flag, Blue bottle, clock, osc clock, paper chromatography, food
coloring in hot, cold water, pH Indicators
2. Sink or
Float?
Ice and water,
p-xylene, bowling ball, cola, diet cola, Al boats,
Galileo's falling rate experiment
3. Hot or Cold?
Liquid nitrogen – balloons (popping and shrinking),
marshmallows, fountain, in water
4. Sound, Light and Space
Vacuum expts- balloon, marshmallow, shaving cream,
light, sound (phone), sun sound vs light,
torch + metal tube
5. Foamed
and Fooled!
Elephant toothpaste, pumpkin, 3 cup Monty
6. Does It Burn?
Cannon, flame tornado, gas bubbles, sterno with colors,
dollar bill
7. Does it glow?
Chemiluminescence, pickle, Hg (fluorescent
screen)
8. Miscellaneous Demonstrations.
Endothermic reaction, smoke rings,
nylon, slime.
This organization of demos was designed for
presentations at the the Great Valley Museum
on the West Campus of Modesto Junior College in Modesto, CA

1. Over the
Rainbow:
The Colors of Chemicals
The images in the collage below taken from the Internet have a common
feature that misrepresents normality in the chemistry laboratory. Have you
figured out what the feature is? As a hint, what color is water?
This simple question is usually not adequately answered by people of all ages
with the most common answer being "clear". Clear is not a color and
is usually incorrectly followed by transparent or white. Whether the color of a liquid
should be described as white is debatable although it is applied to wine (most
"white" wines look yellow to me). Rarely until hints are given for water, someone will suggest the best
answer of "colorless".
If you could form two lines of containers with chemicals, one with liquids
and the second with solids, what would you observe regarding colors of the
chemicals? The vast majority of the liquids would be colorless and the
solids would be white. Most substances do not absorb light in the visible
spectrum. It is common for images of chemicals such as those
in the collage to include predominately liquids and solids with bright
colors. This should be recognized as the exception rather than the rule.
This presents a practical problem when a colored chemical is needed
such as for paint pigments or food coloring. There are limited choices for
colored chemicals and many and even most have properties such as toxicity that make them
undesirable for paint or food coloring. The same
properties that give rise to color are often properties that yield toxicity.
The demonstrations in this section involve physical and chemical changes of
color. The demonstrations are easy to prepare and most can be made in
quantities that enable use for many separate presentations with storage for
months possible. Some of the colored chemicals such as methylene blue
might not be in your stockroom and will need to be purchased.

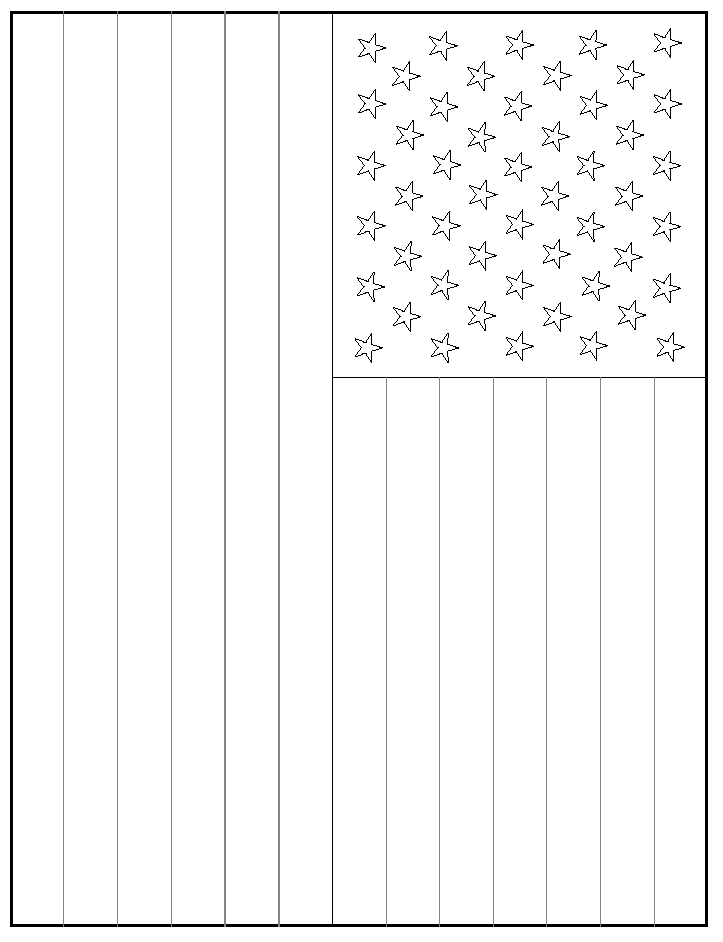
The American Flag. Chemistry
demonstrations that illustrate the importance and excitement of science should
stimulate interest in science and have a positive impact on the science
literacy of the audiences. To increase audience attention and learning and to
dispel the aura of “magic” that sometimes accompanies
chemistry presentations, it is beneficial to involve the audience in
interactive ways during the show. Methods of including audience participation
are presented when appropriate. For the first demonstration
involving colored chemicals, consider the preparation
of an American flag while the audience sings the National
Anthem. For a PowerPoint presentation designed to accompany this demo,
please visit:
http://murov.info/NationalAnthem(U.S.).ppt
or http://murov.info/NationalAnthem/NationalAnthem.html
(no download)
Shortly before the beginning
of the show, a few people are selected from the audience and each is given an
inflated
rocket balloon (or a rocket balloon and an air pump). The audience is asked to stand and at the
count of 3 to sing the Anthem. As the audience starts singing, the “rocket
people” should fully inflate their balloons and hold onto them. When the
singing reaches rockets red glare, the balloon holders should release the
balloons aimed up and toward the back of the audience. When the singing
reaches bombs bursting, the presenter should explode a
carbide cannon or
another safe type of explosive. Finally, when the singing reaches the flag
was still there, the presenter should spray aqueous 2% FeCl3 on a
flag previously prepared with aqueous 5% KSCN stripes and aqueous 5% K4Fe(CN)6 background for the stars. Details for
preparation of the flag and possible alternative chemicals such as the use of
acid base indicators are included below. The
flag demonstration can be used as a teaching moment to emphasize the importance
of observation in science. After singing the Anthem, the audience can be
asked if the Anthem has ever led to the questioning of any of the contents of
the Anthem such as the meaning of the word ramparts. Despite the fact that
the audience has heard or sung the Anthem dozens of times, most people
have overlooked the fact that they don’t know the meaning of ramparts and
could want to improve their observational skills. One of the slides in the
PowerPoint presentation includes a picture of ramparts. It is
interesting to ask and discuss what was meant by rockets red glare when the song
was published in 1814. The audience can also be asked how many are aware that
there are several more verses to the Anthem. At the risk of starting a
political debate, the audience can be asked if it is appropriate for the
Anthem to focus on war instead of peace or the beauty of our country (e.g., see
the 03/02/09 Get Fuzzy comic strip by Darby Conley). Finally, some humor can
be added by informing the audience that a critique of their singing is in
vogue. This can be followed by showing a picture of American Idol’s Simon
Cowell.
General findings included
the following: For paper, Whatman #1 Chromatography paper costs more than
other absorbent paper (46 cm x 57 cm sheet which can be cut
into four 8.5 inch x 11 inch sheets) but a less expensive alternative is
available from Sargent-Welch at
Chromatography Paper | Sargent Welch. Watercolor
and construction papers were easier to paint along lines but spraying usually
resulted in running of most of the colors. The red from 5% aqueous KSCN + 2%
aqueous FeCl3 spray ran the most but acid base indicators phenolphthalein
and thymolphthalein + 0.1 M NaOH spray also ran. Only the blue from 5%
aqueous K4Fe(CN)6 appeared to bind to the watercolor
and construction papers and not run. For large audiences, use of the whole 46 cm
x 57 cm sheet is desirable for adequate visibility but requires much more
preparation time than use of an 8.5 inch x 11 inch sheet (adequate for
audiences of 100 or less). The large sheet requires pencil lining by hand
using template 1 and substantial painting time while the 8.5 inch x 11 inch
sheet can be lined with a printer using the template below and painting time
is relatively short. Alternatives for colors include 5% aqueous KSCN for red
and 5% aqueous K4Fe(CN)6 for blue with spraying with 2%
aqueous FeCl3 or 0.5% phenolphthalein in 95% ethanol for “red”
(quotation marks because the red is reddish-pink) and 0.5% thymolphthalein in
95% ethanol for blue with spraying with 0.1 M NaOH. The acid-base
indicators have the disadvantage that 0.1 M NaOH is caustic and must be
sprayed very carefully away from any people. In addition, the blue lasts only
a few minutes before fading (presumably due to carbon dioxide in the air).
The fading could be used in conjunction with the blue bottle experiment to
discuss the issue of carbon dioxide and global climate change but has the
disadvantage that the fading of the blue contradicts the “flag was still
there” theme of the National Anthem. The K4Fe(CN)6 has the
disadvantage that yellow, starts to appear about a week after painting so
painting should be performed within a few days of use.
For multiple copies of flags, use pencil on
a piece of Whatman 46 cm x 57 cm Chromatography paper or less expensive Sargent
Welch paper
Chromatography Paper | Sargent Welch to draw the lines as
indicated in Template 1 to make a large flag. Technically, the paper does not
have the correct length to width ratio for an American flag but it is easier to use the paper as it comes from
the box. If you choose to cut it to the proper ratio of dimensions, then all
measurements will need to be recalculated. Use Template 1 to mark one flag
and use that marked flag as the template for all future flags. Tape the flag
to a piece of cardboard or mat board before applying the solutions. Painting
should be done at least a couple of hours before use to allow for drying. Use
about a 3/4 inch brush to paint the stripes with 5% aqueous potassium
thiocyanate solution (or 0.5% phenolphthalein in 95% ethanol). Because the
solution will spread about 1/8 inch in both vertical directions, some space
should be left between the application and the pencil lines. For the star
portion of the flag, use a small brush (about 1/4 nch) and paint a frame
with 5% aqueous potassium ferrocyanide (or 0.5% thymolphthalein in
95% ethanol) around the star section. Now paint diagonally in both directions
between the pencil lines leaving intersections of the pencil lines dry. There
also might be a few spots around the edges that need to be filled in with the
potassium ferrocyanide solution. Only one student out of hundreds has
ever complained that the stars that result are distorted circles rather than
stars. After the paper is dry, use a spray bottle to apply 2% aqueous
iron(III) chloride (or 0.1 M NaOH).
For smaller flags, cut the 46 cm x 57 cm sheet Whatman paper into 8.5
inch x 11 inch pieces and run them through a printer capable of dealing with
relatively thick paper using template 2 or 3. Paint as above with a small (0.25
inch) brush.. For the star
portion, paint around the region and then diagonally between the stars making
no attempt to make actual stars.
An alternative to the flag and a less time consuming
preparation involves the spraying of a welcome sign such as
WELCOME MJC.
Blue Bottle
Experiment. The following four solutions are prepared
and stored in plastic bottles and dropper bottles.
Solution A: 32 g
KOH/500 mL water.
Solution B: 40 g dextrose/500 mL water.
Solution C: 0.04
g methylene blue/100 mL water.
Solution D: 1 g resaurzin (tablet form)/100 mL
water.
Mix about 30 mL of solution A, 30 mL of solution B, 10 drops of
solution C and 10 drops of solution D. Stir, allow to sit and turn pink and
then almost colorless (several minutes the first time and shorter amounts of
time later depending on the amount of stirring). Pick up the flask very
carefully and give it one quick swirl to turn it pink. Vigorous stirring will
turn the solution purple. The cycle can be repeated many
times.
A modified blue
bottle experiment should
also be started early and recycled several times before it is discussed. The
solutions are mixed and after several minutes the dextrose in the presence of
base reduces the two dyes to colorless forms but swirling introduces enough
oxygen to reproduce the colored forms. Usually after the solution loses its
color, I ask students to describe their observations. Most will say that the
solution has changed from blue to red to clear. Then I ask for the color of
water and the point is made that clear does not necessarily mean colorless and
that it is very important to be sure that observations are complete and
understandable. Actually there is an additional observation that there is some
color near the top surface but unless you ask some students near the front to
look very carefully at the top surface, most will not see the slight tinge of
color and those that do tend to ignore this extremely important clue. After the
colored, colorless cycle has been repeated about three times, ask the students
to suggest an explanation for the change that occurs upon swirling. Usually the
answers include the words "mixing" or "energy" but occasionally without leading
too much (ask what you could be adding), someone will suggest air or even
oxygen. At this point, you might want to discuss the components of air and most
students will say oxygen, carbon dioxide and rarely nitrogen. I often get their
attention before giving them the top three gases in dry air by telling them they
should bet (no more than $1) their parents that they can not name the top three
gases. If you want to play a little more with this, after naming nitrogen (78%)
and oxygen (21%) and asking how much of a third gas can be present, I write the
following on the board and ask them what it is:
If they
answer "the alphabet", remind them again of the importance of careful and
complete observations. In the sequence of letters the R is missing or the R is
gone hence the sequence represents argon or the third most abundant gas in the
atmosphere (0.93%) (I recognize that some of you are hissing at this point but
that is the expected and desired reaction - humor even if it is kind of
primitive is occasionally needed - for more chemistry puns, see http://murov.info/chemicalpuns.pptx or http://murov.info/PPTPreshows.htm ).
If the audience will stand for more discussion at this time, it is possible to
point out that one of the common answers, carbon dioxide is the fourth gas but
at a much smaller amount (0.04%) and the small percentage is
the reason that humanity has been able to actually increase its value during the
last 150 years by about 46%. This could lead to a discussion of the Greenhouse
effect and the crisis that could confront society if the dire
predictions turn out to be factual. The evidence that the
predictions are true continues to accumulate and are overwhelming (see:
https://www.ipcc.ch/report/ar6/wg1/
)
Clock Reaction. Prepare the following
two solutions in plastic bottles:
Solution A: Dissolve 4 g of soluble starch
in 1 L of boiling water. After cooling, add 2 g of
Na2S2O5.
Solution B: Dissolve 2 g
KIO3 in 1 L of water containing 0.3 mL of concentrated sulfuric
acid.
Mix: 25 mL of solution A and 25 mL of solution B (about 13 seconds for
color change)
The clock
reaction can
be used in several ways. For sophisticated groups one can ask for predictions
about times for diluted systems. The undiluted system as presented takes about
13 seconds for the purple flash of the iodine starch complex. Two-fold dilution
with water leads to about a 55 second period (about 4 times longer as expected). Another variation is to use
boiling water to dilute the mixture. The discussion can be related to the
ink in cold and hot water experiment. With fifth grade audiences, I ask the
classes if they have ever wanted to make noise at school and after their
enthusiastic response immediately qualify the comment to say that they all can
count as loudly as they want but slowly and in unison. Then I predict that the
reaction will take 13 seconds and tell them to start counting at the instant of
mixing. Usually if the counting is slow, the solution will turn within a couple
of counts of 13.
Oscillating Clock
Reaction. Prepare the following solutions in plastic
bottles:
Solution A: Dilute 206 mL of 30% H2O2 to 500 mL with water. [Note:
27% hydrogen peroxide can be purchased under the trade name Baquacil Shock
and Oxidizer from pool stores. See info below.
Solution B: Dissolve 21.4 g of
KIO3 in 500 mL of water containing 2.2 mL of 18 M
H2SO4.
Solution C: Dissolve 3 g of soluble starch in
750 mL of boiling water. To the cooled solution, add 11.7 g of malonic acid
and 2.5 g of MnSO4
.
H2O.
Mix: Equal volumes (for small audiences, 25 mL of each is
sufficient) of solutions A, B, C in an Erlenmeyer flask. While the
demonstration is dramatic in an Erlenmeyer flask, it is even more
impressive if the solution is quickly transferred after mixing to a graduated
cylinder as there is a spatial effect to the oscillations that is easily
observable.
Baquacil® Pool
Oxidizer. From:
MTAS_Use of Hydrogen Peroxide for Coronavirus Disinfection.pdf (tennessee.edu)
This product is a liquid oxidizer that
contains 27% specially stabilized hydrogen peroxide and is used to clarify
swimming pool water.
Care must be taken when handling this product before dilution because a 27%
concentration of hydrogen peroxide can cause eye and mucous membrane irritation,
eye damage, and skin irritation (UN 2014, ERG Guide 140). Use gloves, eye, and
splash protection when handling the concentrated product.
The oscillating
clock reaction (after
mixing the color changes colorless to yellow to purple will reoccur many times
over a several minute time period) naturally follows the clock reaction and is
used mainly for its fascinating properties but it is also used as the basis of a
brief discussion on the infancy of scientific discovery and how much there is
still left to be investigated. Many children think scientists know everything.
The point apparently gets across as students have written: "I know you guys
don't know everything" and "I hope you find out about the ones you don't know."
The word magic can also be discussed here.
To involve the audience, tell the audience to lean collectively to the right and
say ooh when the solution turns dark blue. When the solution turns colorless,
they should lean to the left and say aah. They can relax and sit straight up
when it is briefly yellow. Be sure to let the audience know when they can stop
reacting to the demonstration.
Mellow Yellow Reaction.
The narrative for these reactions is left to you but the audience can be told
that you are trying to make a solution with a mellow yellow color but as you
proceed, the colors keep getting darker. It appears you will not be
successful as how do you change from a dark solution to a yellow one?
| |
A. 30 g iron(III) chloride/100 mL water
B. 22 g ammonium thiocyanate/100 mL water
C. 20 g
tannic acid/20 mL
D. saturated oxalic acid |
Flask 1 - 15 drops of A, Flask 2 - 1 drop of B, Flask
3 - 4 drops of B, Flask 4- 12 drops of C, Flask 5 - 10 mL. Start with 1 L
beaker with 400 mL water. Pour 100 mL into A, then back into beaker,
repeat with each flask. |
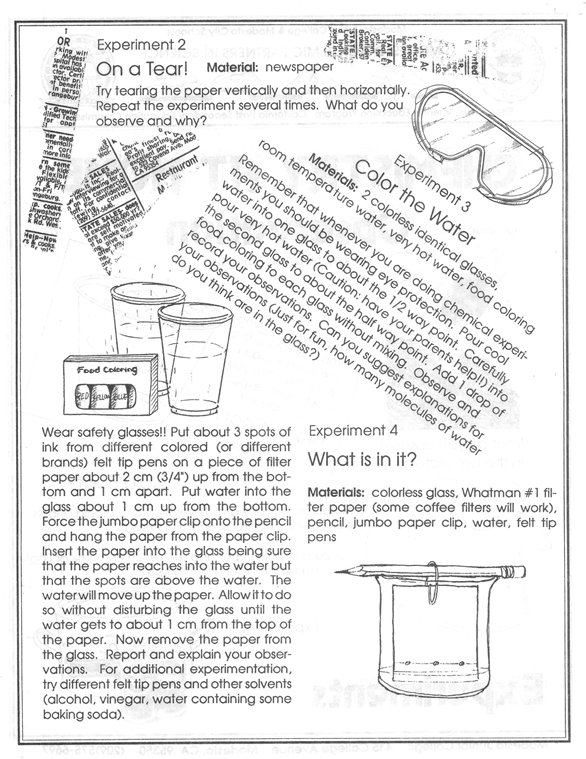
Paper chromatography. Felt
tip pens (try Vis a Vis wet erase felt tip pens) are used to spot a piece of Whatman #1 chromatography paper (or similar) cut appropriately (11 x 19.5 cm) to
fit a 600 mL beaker. The paper is suspended with a large paper clip on a pencil.
When water is used as the solvent, the separation is not complete but sufficient
for students to see separation and differences between different colors and the
same color of different brands. About 40 mL of a 1:1:1 mixture of 1-butanol,
ethanol and 2 M ammonia works much better with some brands and the mixture seems
to be stable for long periods of time. It does require more time for preparation
and it smells.
http://murov.info/EM-st6.pdf
http://murov.info/chemhome.pdf
HOME: This experiment
can be done at home with water, coffee filter paper and food coloring or
appropriate pens.
Molecular Motion. A
drop of food coloring is added to tall 200 mL beakers containing room
temperature and very hot water.
Discussion
of the experiment centers around molecules and molecular size and motion. Models
of molecules such as water, butane, aspirin, penicillin G, and ampicillin ( a
very brief discussion of pharmacology can be brought in here with regard to the
differences between penicillin and ampicillin and why chemists designed and
synthesized ampicillin) are demonstrated. As a hint on the identification of
aspirin, ask the students if they are always good at school or if they ever give
their teachers headaches. Interesting answers are obtained if students are asked
to compare the number of molecules of water in the beaker to the number in a
balloon and to estimate the absolute number. Numbers given will usually be very
low (sometimes starting at 3) until you keep telling them "higher". Be prepared
to explain the meaning of googol and infinity. Solids, liquids, gases and
molecular motion are also compared at this point.
Additional productive discussion is possible if a heater stirrer (without the
word magnetic printed on the front) is used to heat the water. The
audience members are asked to explain the stirring mechanism of the stirrer.
http://murov.info/EM-st14.pdf
Even if it is carefully explained that the heating and
stirring mechanisms are independent, someone will answer that the stirring is
caused by the heating. Others will say vibration and still others, before the
word magnet comes out, will say the white stirrer is a piece of chalk (but would
chalk go around?). The reason for the plastic coating on the magnet can also be
discussed and some students say it is there to trick them. One fifth grader even
came up with an innovative application for the stirrer: "I liked the jar where
you make a whirlpool. I wish I had one of them. My dad would mix Martinis with
it." It is also possible to bring some chemical engineering into this by asking
what properties (melting point, inert) the white plastic should have and can
they think of a plastic with the desired properties.
HOME:
With parental help (pouring the hot water),
this experiment can be done at home.
pH Indicator. Several
similar instructions for the preparation of universal or Yamada indicator exist.
One
of the most common follows:
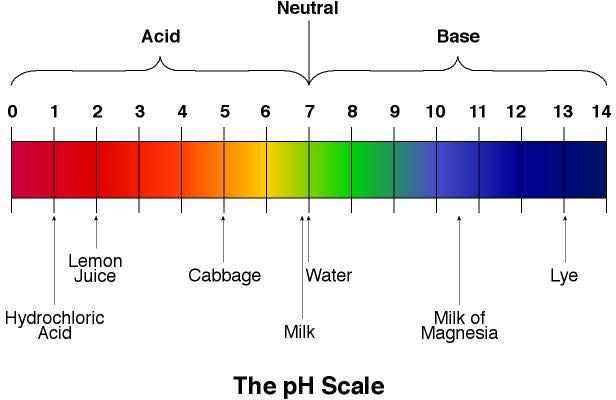
To prepare 1000 mL indicator, dissolve the following amounts in 500 mL ethanol:
0.025 g Thymol Blue,
0.060 g Methyl Red,
0.300 g Bromothymol Blue
0.500 g Phenolphthalein.
Neutralize the solution (to green) with 0.05 M NaOH and dilute to 1000 mL with
water.
The approximate resulting colors are given in the figure to the right:
Depending on the audience size, add several drops of the
indicator to water. Use of a magnetic stirrer facilitates the
demonstration. Now add 0.05 M HCl dropwise until the solution turns reddish.
Add 0.05 M NaOH dropwise with mixing to take the solution slowly from red too
orange to yellow to green to blue to dark blue. This process can be
repeated back and forth as many times as desired.
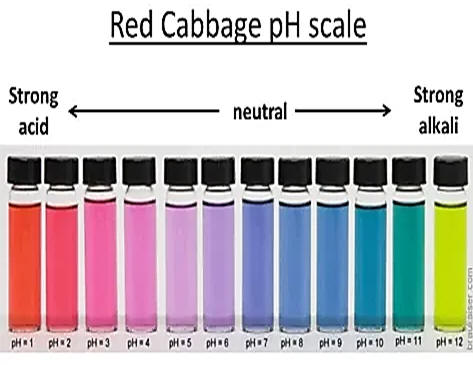 HOME:
To prepare an
indicator at home that will display a few colors at different pH values, place
several leaves of red cabbage leaves
in a small pan and cover with water. Bring
water to a boil and immerse filter paper (some coffee
filter papers will work) into
the now purple colored liquid. Allow the
paper to dry and test with different solutions.
A
HOME:
To prepare an
indicator at home that will display a few colors at different pH values, place
several leaves of red cabbage leaves
in a small pan and cover with water. Bring
water to a boil and immerse filter paper (some coffee
filter papers will work) into
the now purple colored liquid. Allow the
paper to dry and test with different solutions.
A
lternatively, several drops of the cabbage juice can be added to a
solution.
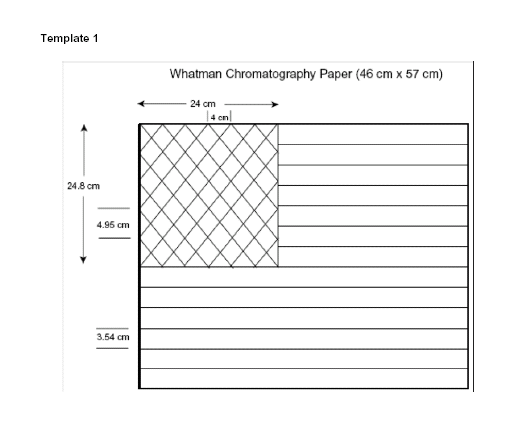
Templates 2 and 3 for 8.5 inch x 11
inch
chromatography paper are on next pages.
fic
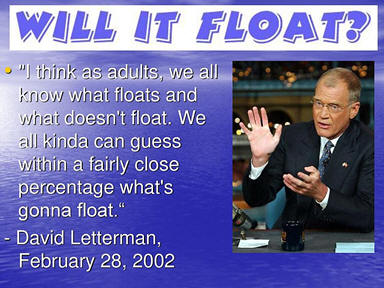
2. Sink or
Float?
Many years ago, David Letterman had a popular segment on his late night
show that asked the question, Does It Sink or Float? Several easily
demonstrated examples surprise many audience members and can help people
understand the concepts involved including the crucial role of density.
Density is the ratio of the mass to the volume. For an object to float,
the density of the object must be less than the density of the liquid. For
objects such as boats, the whole volume of the object needs to be used.
This is the reason that steel ships despite the much larger density of steel
than water have an overall density less than the density of water and float.

Colas and diet colas. Ask the audience if cans of cola and its diet
variety (Coca Cola and Pepsi Cola both work) will sink or float. They will
be surprised that the regular cola sinks meaning that the mass of the can and
contents divided by its volume is greater than the density of water (0.998
g/mL). The diets float and the question is what is the difference.
Since artificial sweeteners are about 200 times sweater than sugar, much less
sweetener is needed and the diet can weighs less than the regular resulting in a
density less than the density of water. If desired, this demo can be used
to emphasize one of the many advantages of the metric system over the American
system. What is the density of water in lb/pint?
Density is a very important
physical property that like melting points and boiling points is very useful for
identification purposes. These properties have a very important attribute
in common, none depend on the amount of substance present. 1 gram of a
substance has the same melting point and density as a ton of the substance.
Density generally requires measurements of both mass and volume. However,
there are tools that are calibrated in density units such as a hydrometer which
can be used to determine the density of car battery fluid (is the battery still
useful?) and car radiator fluid (how low can the temperature go before it
freezes?). Density is a very important property used for the selection of
materials. For example, the density of aluminum is less than half of that
of iron. Other properties such as strength and corrosiveness might also
have to be considered. Titanium with a relatively low density and
high strength might also be considered but price might be a deal breaker.
Bowling balls. Ask an audience if they think a
bowling ball will float or sink. The answer is that it depends on the mass
of the bowling ball. Standard 16 lb bowling balls are denser than water
but bowling balls that weigh less than about 11.5 lbs (5.27 kg) will float.
Bowling balls are available that weigh less than 11.5 lbs.
HOME: Math
problem. A standard bowling ball has a diameter of 8.50 inches. Ignoring
the finger holes, what is the maximum mass in lbs and kg that a bowling ball can
have and still float in water at 20oC?
Water and Ice. A very
common sight is a container with ice and water. Most have observed that the
ice floats in the water. Millions of substances have been characterized (some
properties determined) by chemists. Of the millions, it is interesting and
astonishing to note that almost all of us have seen the liquid and solid phase
of the same substance in the same container only for the substance, water. In
other words the question of whether the solid sinks or floats in its own liquid
is observed by most for water only. As a result, the behavior of water is
accepted by most as the norm. However, think about this. As the liquid
condenses, it should contract in volume until it freezes where further
contraction is intuitively expected. Thus the solid is expected to be denser than its liquid and sink in the liquid. Except for water and a few other
substances (including the elements silicon, germanium galium, arsenic and
bismuth), th is expectation is realized and the solid sinks in its liquid state
(consider what would happen to lakes in the winter if water behaved like almost
all other substances). Observation is the key to good science. A
good observer would ask the question why does the ice float but very few of us
have asked this question and we need to learn to make more complete
observations. The question why ice floats in liquid water (why ice is less dense
than liquid water) is a difficult one but water molecules are closer together in the liquid state than in the
solid state
For more information, please visit: http://murov.info/EM-st1.pdf
. To demonstrate that water is unusual, the easiest comparison is to place
ice in water and p-xylene in another test tube. Place the
p-xylene test tube in an ice water bath for a few minutes and scratch the inside
of the test tube with a glass rod. Some of the p-xylene
should freeze and the solid as contrasted with the water test tube should sink.
Other substances can also be used but p-xylene is preferred.
is expectation is realized and the solid sinks in its liquid state
(consider what would happen to lakes in the winter if water behaved like almost
all other substances). Observation is the key to good science. A
good observer would ask the question why does the ice float but very few of us
have asked this question and we need to learn to make more complete
observations. The question why ice floats in liquid water (why ice is less dense
than liquid water) is a difficult one but water molecules are closer together in the liquid state than in the
solid state
For more information, please visit: http://murov.info/EM-st1.pdf
. To demonstrate that water is unusual, the easiest comparison is to place
ice in water and p-xylene in another test tube. Place the
p-xylene test tube in an ice water bath for a few minutes and scratch the inside
of the test tube with a glass rod. Some of the p-xylene
should freeze and the solid as contrasted with the water test tube should sink.
Other substances can also be used but p-xylene is preferred.
Extension: Try many other household items. Ask the audience before
testing whether the item will sink or float. Suggestions: apple,
orange, avocado, raw potato, baked potato, hollow steel ball and solid steel
ball. It is also interesting to pass out a small ball bearing, a rubber
stopper and a cork ring and ask the audience to rank them by increasing mass
(see: http://murov.info/EM-st7.pdf
). While this hands-on exercise is not about density, it does
demonstrate that we have to be careful with preconceived notions. In this
case, the hand probably perceives pressure or mass/area2.
Rate of Falling:
Although it has been demonstrated that the rate of falling in a vacuum does not depend on
the mass or density of the object centuries ago, it is likely that many people
think that mass does influence dropping rate. To definitively demonstrate
this would be difficult without extensive instrumentation, the dropping of two
same sized plastic bottles, one filled with a dense substance such as iron or
lead and the other empty, should appear to hit the ground simultaneously if
dropped from about 5 feet. The density of the empty bottle is much less
than the density of the filled bottle but within observational limits, the two
bottles fall at the same rate. For more information on this probably
counterintuitive concept, please visit:
The legend
of the leaning tower – Physics World
and
Galileo's Leaning Tower of Pisa experiment - Wikipedia
HOME or in group activity: Aluminum boats. Each participant is
given a 6 or 8 inch square piece of aluminum foil. The aluminum is folded as
desired to make a boat. The boat is floated in a tub of water and the
number of pennies needed to sink the boat is determined with the most pennies
added determining the winner.
3. Hot or Cold?
Liquid
Nitrogen.
Liquid
nitrogen is available in many locations but for most presenters, requires the
extra effort to get it (compressed gas sources sometimes stock it). For
occasional use, a 4 Liter Dewar is recommended (available for $100 to $1000).
If presentations are given frequently, a larger container such as a 40 liter
Dewar is suggested (around $1000). The price of the liquid nitrogen will
vary with the source but should not be more than $5/L. The presentation
can be started out by stating that a mysterious substance in the vacuum flask
will be used for several experiments during the course of the presentation
(about one every 10 minutes). The students are asked to remember their
observations and hopefully deduce the properties (is it hot, room temperature,
or cold, is it solid, liquid or gas, is it colored or colorless) and the name of
the substance by the end of the program.
1. A balloon is attached to a piece of
vacuum hose with a hose clamp. The other end of the hose is connected to a
plastic bottle containing a small amount of liquid nitrogen.
2. An inflated
sealed balloon is slowly pushed into liquid nitrogen. Balloons that have bulges
or twists are more interesting. After the balloon has shrunk to a minimal
size, it is allowed to warm to room temperature. For college audiences, ask
why the volume decreases to close to zero rather than about 1/4 of the volume
as predicted approximately by Charles’ Law.
3. After liquid nitrogen is added
to a plastic bottle, a rubber stopper is securely inserted into the
bottle. After several seconds it will shoot out into the audience if aimed
properly.
4. Into the plastic bottle containing liquid nitrogen, insert a
rubber stopper with a 25 cm long piece of 7 mm tubing that reaches to near
the bottom of the bottle. A fountain will result and if sufficient nitrogen
is used, it is possible to almost disappear in the descending fog. The rubber
stopper should be held carefully as on rare occasions, liquid nitrogen runs
down the glass tube and can freeze small portions of your fingers.
5. A
marshmallow (or many other interesting objects) is placed on a copper wire and inserted into liquid nitrogen. After
about 3 minutes, gentle tapping in a beaker will shatter the
marshmallow. One alternative is to smash the marshmallow with a hammer.
6. Liquid nitrogen can be harmlessly poured quickly on the back
of your hand for very short periods of time.
7. Have the children separate
if the floor is carpeted and leave a wide aisle and throw some
liquid nitrogen onto the floor between them.
After seeing the marshmallow, students will respond with great enthusiasm if you
ask them if they want you to put your hand in the liquid nitrogen and bang it on
the table (don't do it but as indicated above you can pour a small amount
carefully onto the back of your hand). One student wrote: "Next time you make an
icemallow, taste it and tell me what it tastes like. But if it's poisonis and
you die don't tell me. If you ever shater your hand in liquid nitrogen, I hope
you have some glue with you." Students have a difficult time grasping the liquid
air concept as they do not generally understand the solid, liquid and gaseous
states. The system can be related to ice, water and steam but difficulty is
still encountered. The teachers should probably discuss the liquid nitrogen
demonstration in detail in their classrooms.
4. Sound, Light and Space
A vacuum pump is needed for these demonstrations but relatively inexpensive
ones that should be adequate are available under $100 from sources such as
Harbor Freight. Also needed is a plastic bell jar. These are
available over a big price range but a reasonably sized one should be available
for under $200.
Experiments
in the vacuum chamber are used to demonstrate the effects of a vacuum on
marshmallows, slightly inflated balloons, shaving cream and sound. sources.
1. Slightly inflate and tie off a small balloon and suspend it from
the top of the chamber with a short piece of tape. Evacuate the chamber.
The audience is asked to name 3 methods of inflating a balloon (adding a
substance such as air or water to the balloon, heating the balloon, removing the
air from the exterior of the balloon). The third reason is not trivial for
some people to understand. If the balloon has been inflated properly, it
will eventually pop but the sound intensity will be much less than if air had
been in the chamber. If the audience is asked to give their observations,
seldom is the intensity of the popping mentioned. The importance of
complete and unbiased observations can be discussed. When asked why
popping was relatively quiet, the most common answer is that the chamber
insulates the sound. While this does have an effect, it is a minor one
compared to the fact that sound cannot travel in a vacuum. A short
discussion of sound follows with the audience asked to cover their larynx and
talk and notice the vibrations. A little sound is heard because the
balloon is usually touching the side of the chamber. Other experiments
included below reinforce the inability of sound to travel in a vacuum. It
is also useful to compare sound waves to light waves. With a clear and
colorless chamber, the audience should be able to observe that light travels
through the chamber. A light source such as a flashlight can be used to
emphasize this. The relative speeds of sound and light can also be
discussed. To illustrate the point that light travels through a vacuum but
sound does not, discuss the waves that reach us through a vacuum from the sun.
What kind of sound does the sun make?
2. Insert 2 or 3 large marshmallows into the chamber and evacuate.
Audiences are even more fascinated if marshmallow shapes such as chicken or
bunny Peeps are used.
The marshmallows expand substantially due to the trapped air and
reach a maximum and then shrink back a slight amount presumably due to the
popping of some of its cells. When the air is readmitted to the chamber,
the marshmallows shrink to a size much smaller than their original size.
Ask the audience if the marshmallows should be eaten at this point and many will
say no primarily due to their appearance. Although the texture has changed, the taste would probably not be
significantly changed but a discussion of the safety of eating food
that has been in a laboratory environment.
.3. Use a can of shaving
cream to place a small amount of shaving cream in the bottom of the chamber.
As a vacuum is established, the shaving cream will expand to fill the chamber
but this does require time for cleaning.
4. Other devices that make sound can be placed in the chamber such as
a mechanically wound alarm clock or even a cell phone. They should be
suspended so that they are not touching the sides and the sound they produce
should be demonstrated first with air in the chamber.
5.
Attach the vacuum pump via a hose
connected to the appropriate size single hole rubber stopper to a large plastic
bottle and afterwards to a 5 gallon metal can (empty acetone cans that have been
thoroughly rinsed with water usually work). Generally, collapse after a
couple of minute of pumping occurs in a
very dramatic fashion. This demo has been use by the group Weird Science
to amazingly collapse 55 gallon drums.
6. There are many sound demos available but an intriguing one involves a
thick metal pipe available as a singing pipe from
https://www.teachersource.com.
A
good heat source such as a portable propane torch is used to heat the air near
the end of the tube that contains a metal screen, the tube will whistle when
held vertically but temporarily will go quiet when rotated to a horizontal position.
This is repeatable.
5. Foamed
and Fooled!
Elephant Toothpaste.
Elephant toothpaste is a great crowd pleaser but be prepared for
cleaning up a mess. This reaction can be run in various ways and at
different scales. A standard demonstration uses about 100 mL of approximately
30% hydrogen peroxide (see notes under oscillating clock reaction) in a 250 mL graduated cylinder. Add to the solution
about 10 mL of Joy or Dawn detergent and several drops of food coloring as
desired. Place the cylinder in a dish tub and add about 7 mL of 2 M NaI
(or KI) and back up. The foam that results will quickly overfill the tub.
Any unreacted H2O2 will bleach hands white so avoid contact with the reagent or
the foam. It is also possible to use a bbq lighter to try to ignite the
foam. The audience expects a big flame but all that is noticed is that the
flame gets a little brighter due to the high percentage of oxygen in the foam.
An alternative and more impressive demonstration results with the Weird Science
technique of running the reaction in a carved pumpkin. Introduce the
peroxide, detergent and food coloring into a 200 mL beaker placed in the bottom
of the pumpkin. Add the NaI solution and quickly close the top of the
pumpkin. The foam will emerge from the openings of the pumpkin. To
partially contain the mess, mount the pumpkin on an inverted dishpan with a
dishpan in front of the inverted pan. Much but not all of the foam will
end up in the front dishpan.
3 Cup Monty.
Add water to an opaque container that has
powered sodium polyacrylate (available from
https://www.teachersource.com or
Amazon, etc.) in it. Turn the container upside
down and nothing comes out. Add salt and stir and the aqueous phase can be
poured again. Mention the role of the powder in disposable diapers to make the
discussion relevant. An alternative is to play the 3 cup Monty with the audience. Put a small amount of sodium polyacrylate
in an opaque cup before the audience enters the room. If desired, add some water
to a 2nd cup before the audience enters the room. Rapidly switch the
positions of the cups and the ask the audience which cup has the water in it.
Before revealing that most of the audience followed the movement correctly, pick
up the empty cup and take it into the audience and ask if anyone wants it dumped
on their head. Then invert the empty cup over the volunteer's head.
Next pick up the cup that unknown to the audience had water added earlier and
pour it into the sodium polyacrylate cup and swirl. Tell the audience to
be careful about making assumptions and that you had added the water earlier.
Finally, take the cup the audience concluded has the water and add the contents
to the sodium polyacrylate cup and swirl and tell the audience they had
correctly followed the cup with water. Without revealing the contents of
the sodium polyacrylate cup, tell the audience that all the water is now in one
cup and you are going to repeat the experiment. Upon switching the cups
around, take the
two empty cups into the audience and attempt to dump them on "volunteers."
Now take the cup with the sodium polyacrylate gel into the audience and slowly
invert it over a final volunteer. If done properly, nothing will come out
of the cup and you can hold the cup horizontally and show the audience that the
water in the form of a gel is in the cup. You can (preferably with gloves)
reach into the cup and grab a handful of gel and show them what it is and
mention the diaper application as well as use as a source of water for plants.
6. Does It Burn?
Combustion experiments are usually part of most chemistry
demonstrations with dramatic experiments including the exploding of hydrogen
balloons. Except for the popping of balloons from over-inflation, this set of demos avoids the use
of potentially dangerous reactions that from our experience some children have been known to try to
reproduce at home.
1. As suggested in the American Flag demo, when
"bombs bursting" comes up in the Anthem, a cannon can be use to produce a loud
bang. The cannons are somewhat pricey buy available at BIG-BANG
Cannons Conestoga Company (bigbangcannons.com) for under $200.
While calcium carbide is available at low cost, the Conestoga Bangsite
variety (Bangsite) works better than the cheaper stuff. The advantages of the cannon are that appropriate timing is
easy and safety is assured. The bang is produced by dropping a small
amount of calcium carbide into water in the cannon followed by sparking of the
acetylene produced. Audiences often want the bang repeated but generally a
quick 2nd try results in more flame than bang as the needed amount of oxygen in
the cannon has been depleted.
2. If methane (natural gas) is available, with a
tube (containing
a small funnel) connected to the methane source
, it is possible to produce methane bubbles. The bubbles can be ignited with practice to
produce a flame ball. The visual effects are enhanced if this can be done
in a dark room. Bubbles produced by blowing are also tested with a flame
and it should be observed that the blown bubbles go down whereas the methane
bubbles rise. Before revealing the contents of the methane bubbles, it is
possible to contrast the properties of the gases in the two sets of bubbles (the
blown bubbles have higher density than air (soap + a little higher carbon
dioxide concentration) and do not burn while the methane bubbles are less dense
than air and burn. Propane containers for bbq's are available at a much
lower price than methane cylinders but the bubbles are denser than air and sink
and ignition is more difficult and probably not quite as safe.
3. Canned Heat (Sterno). In a Pyrex Petri dish,
put a few mL of saturated aqueous calcium acetate solution (about 35g/100 mL).
Add ethanol, mix, and pour off the excess alcohol from the gel formed. Ignite
with a match and sprinkle some boric acid or other colored flame producers
(e.g., strontium compounds) on the flame.
 4. Flame
tornado. This demo requires some preparation and is somewhat touchy.
A turntable that can be manually spun at a high rate is needed. Also a
wire screen (about quarter inch mesh in the form of a cylinder about 3 feet high
is also needed. A source for a long
burning flame is needed in the center of the turntable that is not capable of
spreading the fire. We use a cotton ball soaked with a color producing
chemical such as boric acid or strontium chloride. The ball is ignited in
the middle of the turntable, and the cylindrical screen is placed on the
turntable in a fixed position (we use a centrifuge for the turntable and
make the screen the right diameter to fit securely on the centrifuge.
With the rapid spinning of the turntable, the flame should form a tornado that
is more impressive in the dark. Pictured to the right is Steve Spangler
performing the demo. The first time I saw this was about 1992 with the
late great Tik Liem doing the demo.
4. Flame
tornado. This demo requires some preparation and is somewhat touchy.
A turntable that can be manually spun at a high rate is needed. Also a
wire screen (about quarter inch mesh in the form of a cylinder about 3 feet high
is also needed. A source for a long
burning flame is needed in the center of the turntable that is not capable of
spreading the fire. We use a cotton ball soaked with a color producing
chemical such as boric acid or strontium chloride. The ball is ignited in
the middle of the turntable, and the cylindrical screen is placed on the
turntable in a fixed position (we use a centrifuge for the turntable and
make the screen the right diameter to fit securely on the centrifuge.
With the rapid spinning of the turntable, the flame should form a tornado that
is more impressive in the dark. Pictured to the right is Steve Spangler
performing the demo. The first time I saw this was about 1992 with the
late great Tik Liem doing the demo.
5. Cold flame. Soak a dollar bill (a larger
amount is a bit of a gamble as the dollar sometimes ignites if the experiment is
not done correctly) is soaked in a 1:1 water to isopropanol
or ethanol solution. The dollar when ignited will burn with a
difficult to observe blue
flame but the paper bill itself should not ignite. It is more impressive
if
salt is added to the mixture first as the flame will be yellow and much easier
to observe. It is possible to
involve the audience by asking for a dollar bill.
7. Does It Glow?
Chemilumescence. Many variations of chemiluminescence
with luminol are available on the Internet and in chemistry demonstration
resources. This is one of the simplest that does not require much
preparation time and the solutions once prepared can be used for
many presentations.
Prepare
the following solutions in plastic bottles:
Solution A: Dissolve 2 g of luminol (aminophthalhydrazide) and 2.5 g of
NaOH in 1 L of water.
Solution B: 0.15%
H2O2
Mix: 40 mL each of solutions A and B in a graduated cylinder.
Sprinkle a few crystals of K3Fe(CN)6 into the cylinder
for an interesting effect and then add a larger quantity for maximum light
output. For a yellow emission, add, after the blue emission is observed, a few
drops of a solution of 1 g of fluorescein and 1 g NaOH in 100 mL of
water.
Fluorescence
and Phosphorescence. Fluorescence can be conveniently demonstrated by
writing on Whatman No. 1 filter paper with solutions containing 0.02% methanol
solutions of Rhodamine B (caution - suspected carcinogen), fluorescein, or
acridine orange. For phosphorescence, write on the filter paper with a
5x10-3 M solution of a polynuclear aromatic acid ( e.g., naphthoic
acid, naphthalene sulfonic acid) in a 1 M NaOH solution. 2-Naphthol gives both
fluorescence and phosphorescence under these conditions. After drying irradiate
with the 254 nm line of a mineral light in a dark room.
Mercury shadowgraph.
ALERT Inhalation of mercury vapor is an extreme health hazard. If this
demo is performed, the mercury needs to be in a sealed container that can easily be opened for a very short period of time. Fluorescence
alone is demonstrated first using a pre-coated
thin layer chromatography sheet with a fluorescent indicator. A short wave (254
nm) mineral light (or chromatography light) is a convenient portable light source. Next, a shallow plastic
bottle of mercury is opened and placed between the light and the fluorescent
screen. In a sufficiently darkened room, the shadows of the mercury vapor are
easily observed on the screen. A little blowing on the mercury sometimes
enhances the demonstration.
While the amount of mercury vapor visible cannot be
estimated from the observation, it is clear that the mercury is
evaporating at an alarming rate (but only about 1/10,000 the rate of water
evaporation). Since the 254 nm from the mineral
light is a mercury emission line, it exactly matches the absorption line of the
mercury vapor.
Electric Pickle. This experiment
should be performed in a well ventilated area or for a very short time only. Cut
off the female end of an inexpensive extension cord and split the wires. Solder large
sheet metal screws to each of the wires. Support a large dill pickle in some way
that won't require touching (we use a ring stand and a clamp)
and insert the sheet metal screws into opposite ends of the pickle. Plug
the cord directly into a multiple outlet with an on off switch and after several seconds smoke should start
coming out of the pickle and shortly thereafter, it should start glowing (and
burning and giving off a nasty odor). At
this point, the lights should be turned out but remember to run this for a short
period of time and be very careful not to electrocute yourself. Students should
definitely be cautioned not to try this one at home. It is possible to use
several pickles simultaneously and use them on a Chemistree.
(S. L. Murov, J.
Chem. Ed., 71,
1082 (1994). "Chemistree" ,
A Chemistree | Journal
of Chemical Education (acs.org)).
8. Miscellaneous Demonstrations.
Endothermic
Reaction.
Alert: Barium is a very toxic ion and care must be taken with disposal. Vials containing 20 g of Ba(OH)2.8H2O. and 10
g of NH4SCN (other less toxic chemicals can be substituted such as Sr(OH)2.8H2O and/or NH4Cl but the reaction is
not quite as dramatic). The formation of water and frost can be observed and the
presence of ammonia detected by smell. The flask gets cold enough
(-20oC) to condense moisture form the air. The condensation can be
demonstrated by rubbing some of the frost on the flask exterior with a finger. Some students near the
front can be allowed to touch the flask and tell the other students that it is
cold. Some students assume all chemical reactions evolve heat and confuse the
frost with steam. The flask gets cold enough to freeze water added to the bottom
of the flask and the flask can be frozen to a smooth surface using ice as
glue.
The importance of the use of all the senses (except
taste) can be stressed when the solids barium hydroxide octahydrate and ammonium
thiocyanate are mixed. This
endothermic
reaction
produces Ba2+, SCN-, water and ammonia.
Sometimes, I ask the students to close their eyes before I mix the chemicals and
tell me what they think has happened just from the change in the swirling
sounds. Students will quickly pick up the solid-to-liquid phase change and if
you allow them a quick whiff, some will recognize the smell of ammonia. One
student had some interesting spelling in the following comment: "Espally I liked
when he truned some stuff into pneumonia." Note the correct spelling of the
incorrect concept.
Returning the to observations, most will not report
the formation of frost on the exterior of the flask and about half say they
think that the flask is hot (many associate chemical reactions with heat
evolution). One can let them touch the outside of the flask, read an inserted
thermometer (about -10oC to -15oC), and/or freeze an
object to the flask by adding water externally. Discussion can follow on
observations, the Celsius temperature scale (let the students invent i
t
if they are not familiar with it), reaction energetics
(including entropy if desired for high school groups, melting points and the
effects of additives on melting points (why doesn't the material in the flask
freeze if it is -15oC?). The salting of roads in the winter and the
preparation of ice cream and antifreeze mixtures for cars can be discussed.
Nylon. In a glass
bottle, prepare a 0.25 M adipyl chloride solution in cyclohexane. Transfer about
20 mL of this solution to a plastic dropper bottle. In a plastic bottle,
prepare an aqueous solution containing 0.5 M 1,6-diaminohexane and 0.5 M
NaOH. Put about 2 mL of the amine solution in a shallow dish or watch glass.
Insert a copper wire with a small loop at its
end into the solution and slowly pull out the nylon.
| Slime |
500 mL water in 1L beaker, green food coloring, 100 mL1% polyvinyl alcohol,
10 mL 4%
borax |
Add food coloring and
borax and mix. |
Smoke
rings |
Smoke machine,
garbage can with 6 inch hole on closed end and flexible
plastic fastened to
cover
other
end |
Shoot smoke rings out into audience |
Some references
Chen, P. S., Entertaining and Educational Chemical
Demonstrations, Chemical Elements Publishing Co., 1974, 20.
American Flag Proportions, http://www.montney.com/flag/proportions.htm (accessed 03/05/09).
For example, see: The
National Anthem: The Star-Spangled Banner,
http://murov.info/NationalAnthem(U.S.).ppt
http://www.usflag.org/thenationalanthem.html (accessed 03/06/09), The Star Spangled Banner
http://www.scoutsongs.com/lyrics/starspangledbanner.html (accessed 03/06/09), Star Spangled Banner.
visitor number started
8/11/21, site posted 8/11/21, last update 8/11/21
Unique visitors started 8/11/21

 http://www.thecatalyst.org/m05demos.html
http://www.thecatalyst.org/m05demos.html 









 4. Flame
tornado. This demo requires some preparation and is somewhat touchy.
A turntable that can be manually spun at a high rate is needed. Also a
wire screen (about quarter inch mesh in the form of a cylinder about 3 feet high
is also needed. A source for a long
burning flame is needed in the center of the turntable that is not capable of
spreading the fire. We use a cotton ball soaked with a color producing
chemical such as boric acid or strontium chloride. The ball is ignited in
the middle of the turntable, and the cylindrical screen is placed on the
turntable in a fixed position (we use a centrifuge for the turntable and
make the screen the right diameter to fit securely on the centrifuge.
With the rapid spinning of the turntable, the flame should form a tornado that
is more impressive in the dark. Pictured to the right is Steve Spangler
performing the demo. The first time I saw this was about 1992 with the
late great Tik Liem doing the demo.
4. Flame
tornado. This demo requires some preparation and is somewhat touchy.
A turntable that can be manually spun at a high rate is needed. Also a
wire screen (about quarter inch mesh in the form of a cylinder about 3 feet high
is also needed. A source for a long
burning flame is needed in the center of the turntable that is not capable of
spreading the fire. We use a cotton ball soaked with a color producing
chemical such as boric acid or strontium chloride. The ball is ignited in
the middle of the turntable, and the cylindrical screen is placed on the
turntable in a fixed position (we use a centrifuge for the turntable and
make the screen the right diameter to fit securely on the centrifuge.
With the rapid spinning of the turntable, the flame should form a tornado that
is more impressive in the dark. Pictured to the right is Steve Spangler
performing the demo. The first time I saw this was about 1992 with the
late great Tik Liem doing the demo.