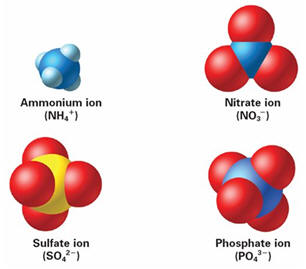
 1. ION
Names and formulas by charge
1. ION
Names and formulas by charge 1. ION
Names and formulas by charge
1. ION
Names and formulas by charge
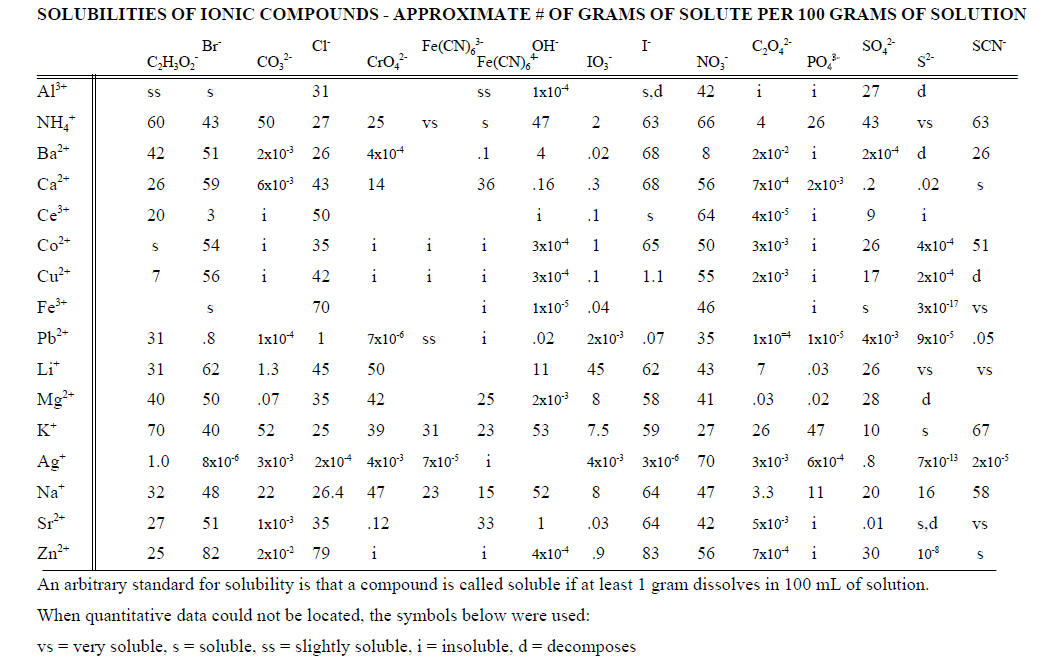
2. Solubilities of ionic compounds in water
3. Anions, Oxoanions and Polyatomics Arranged by Name
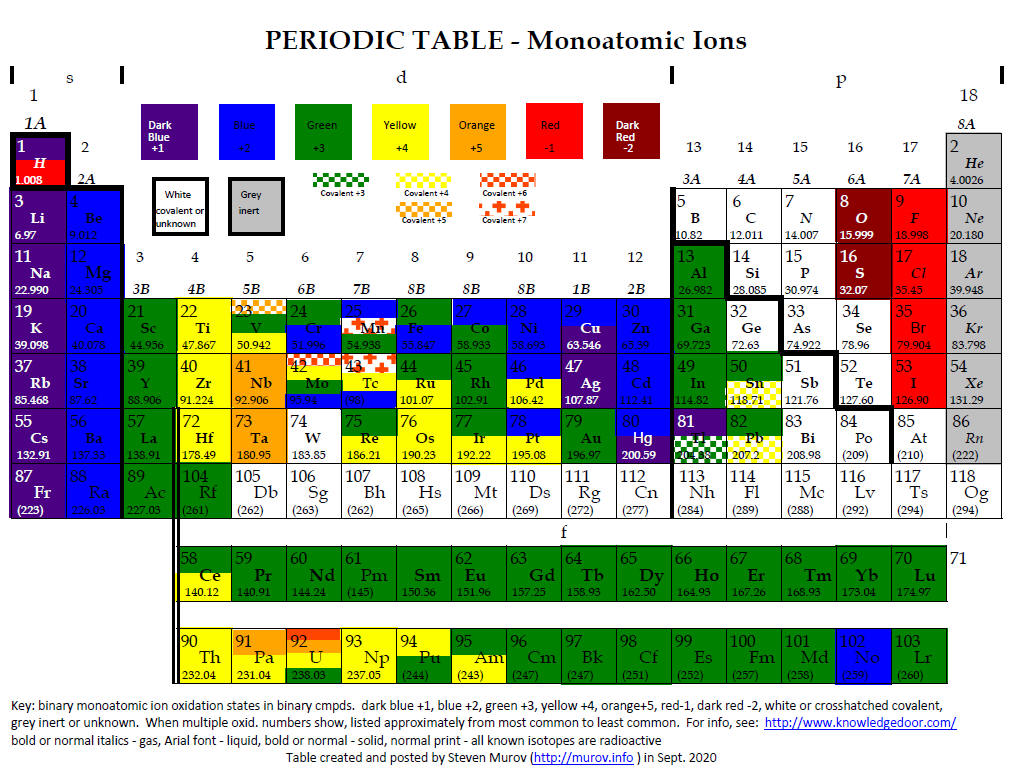
4. Periodic Table of Oxidation States of Monoatomic Ions
For periodic tables including periodic trends and
information on ions and metal vs non-metal properties, please visit:
http://murov.info/periodictables.htm
http://murov.info/pertabtrends.htm
http://murov.info/pertabtrends.pptx
1. iON NAMES AND FORMULAS BY CHARGE
A. Positive Ions
| + 1 | |||
| Ammonium | NH4+ | Mercury(I) (mercurous) | Hg22+ |
| Copper(I) (cuprous) | Cu+ | Potassium | K+ |
| Hydrogen | H+ | Silver | Ag+ |
| Hydronium | H3O+ | Sodium | Na+ |
| Lithium | Li+ | ||
| + 2 | |||
| Barium | Ba2+ | Magnesium | Mg2+ |
| Cadmium | Cd2+ | Manganese(II) (manganous) | Mn2+ |
| Calcium | Ca2+ | Mercury(II) (mercuric) | Hg2+ |
| Cobalt(II) (cobaltous) | Co2+ | Nickel(II) (nickelous) | Ni2+ |
| Copper(II) (cupric) | Cu2+ | Strontium | Sr2+ |
| Iron(II) (ferrous) | Fe2+ | Tin(II) (stannous) | Sn2+ |
| Lead(II) (plumbous) | Pb2+ | Zinc | Zn2+ |
| + 3 | |||
| Aluminum | Al3+ | Cerium(III) (cerous) | Ce3+ |
| Antimony(III) | Sb3+ | Chromium(III) (chromic) | Cr3+ |
| Arsenic(III) | As3+ | Iron(III) (ferric) | Fe3+ |
| Bismuth(III) | Bi3+ | ||
| + 4 | |||
| Lead(IV) (plumbic) | Pb4+ | Tin(IV) (stannic) | Sn4+ |
| + 5 | |||
| Antimony(V) | Sb5+ | Bismuth(V) | Bi5+ |
| Arsenic(V) | As5+ |
B. Negative ions
| - 1 | |||
| Acetate | C2H3O2- | Hydrogen sulfate (bisulfate) | HSO4- |
| Bromate | BrO3- | Hydrogen sulfite (bisulfite) | HSO3- |
| Bromide | Br- | Hydroxide | OH- |
| Chlorate | ClO3- | Hypochlorite | ClO- |
| Chloride | Cl- | Iodate | IO3- |
| Chlorite | ClO2- | Iodide | I- |
| Cyanate | NCO- | Nitrate | NO3- |
| Cyanide | CN- | Nitrite | NO2- |
| Fluoride | F- | Perchlorate | ClO4- |
| Hydride | H- | Permanganate | MnO4- |
| Hydrogen carbonate (bicarbonate) | HCO3- | Thiocyanate | SCN- |
| - 2 | |||
| Carbonate | CO32- | Sulfate | SO42- |
| Chromate | CrO42- | Sulfide | S2- |
| Dichromate | Cr2O72- | Sulfite | SO32- |
| Oxalate | C2O42- | Tetrathionate | S4O62- |
| Oxide | O2- | Thiosulfate | S2O32- |
| Persulfate | S2O82- | ||
| - 3 | |||
| Ferricyanide | Fe(CN)63- | Phosphate | PO43- |
| - 4 | |||
| Ferrocyanide | Fe(CN)64- | ||
| 2. |
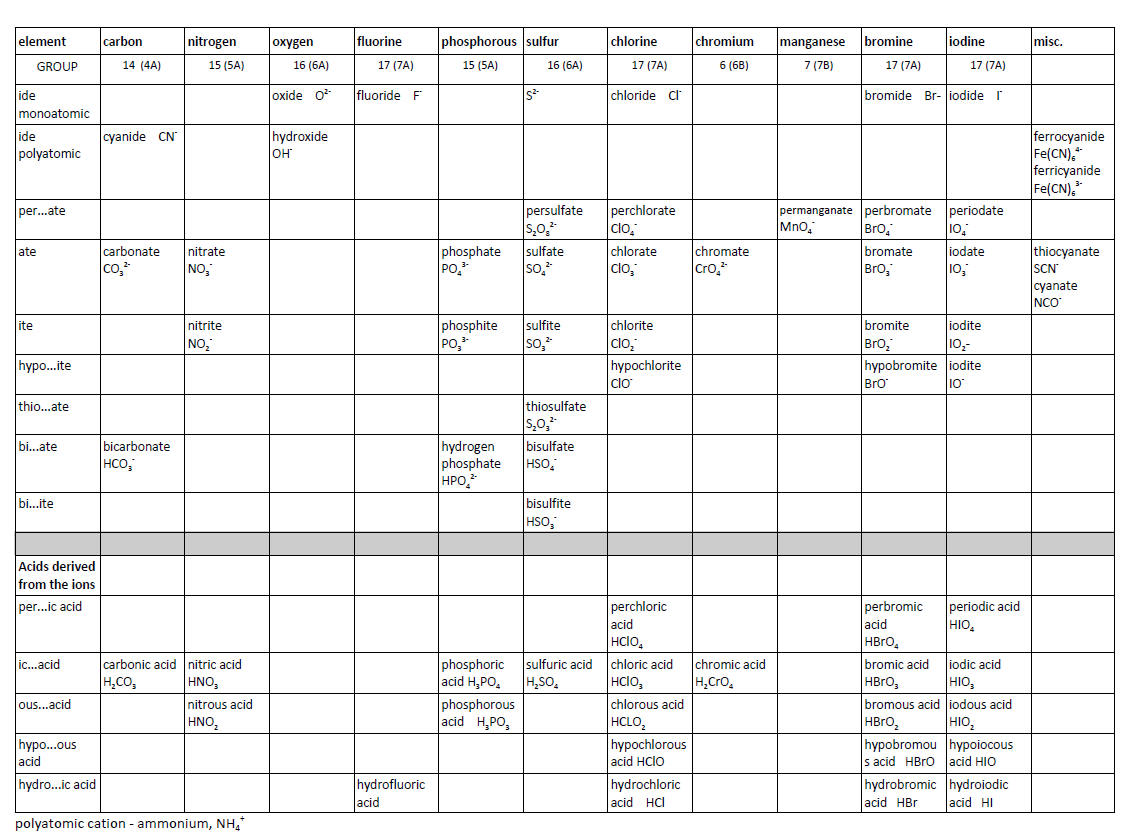
3. Anions, Oxoanions and Polyatomics Arranged by Name
restarted at zero on 07/27/12 contact Steve Murov (murovs@yosemite.edu ) to make comments or suggestions
UNIQUE VISITORS STARTED ON (also solubility chart added) 4/28/20