Medium-long forms of the periodic table with element colors.
A limited search for a periodic table with the colors
of the elements has not yet located a table of this type. However, Theodore Gray
has produced many wonderful tables including one with images of authentic
samples of the elements (http://periodictable.com/) and a commercially
available model that contains sample of most of the elements (http://www.periodictable.co.uk/ ). While the tables below are not nearly as
fascinating or interesting as Gray's tables, the tables below have been designed
to enable viewers to focus on the property of color. Most of the colors have
been extracted from https://www.chemicool.com/ primarily because it often
included a two word description of the color. Other sites referred to were
Theodore Gray's http://periodictable.com/Properties/A/Color.html and Mark
Winter's https://www.webelements.com/ . For some elements, the colors listed on
Internet sites are not always in agreement. Rene Vernon, the author of a paper
on metalloids, https://pubs.acs.org/doi/pdfplus/10.1021/ed3008457 has
contributed valuable comments about the colors of boron, phosphorus, iodine,
cesium and astatine. Some of these color issues are because the most stable
allotrope (e.g., phosphorus) is not always the most abundant allotrope.
boron - the most stable allotrope of boron is the beta rhombohedral crystalline
state. Colors reported for this state range from shiny silver-grey to grey to
dark to black. It is represented as dark grey in the table.
phosphorus - the most stable allotrope is black but the most common form is
described as white to pale yellow. A very light yellow has been used below with
a black insert.
iodine - while silver is sometimes mentioned, the overwhelming consensus is that
iodine crystals are in the violet or purple range.
astatine - although astatine has been observed, due to its transient existence,
it has apparently not been possible to determine its color. Some web sites
conclude that it should have some metallic properties and as a result have a
silvery color. Other web sites suggest as progression is made down group 7A
(17), the color continuously darkens with a presumption that astatine should be
near black. It is left in these tables as unknown like francium and the elements
with atomic numbers above 99.
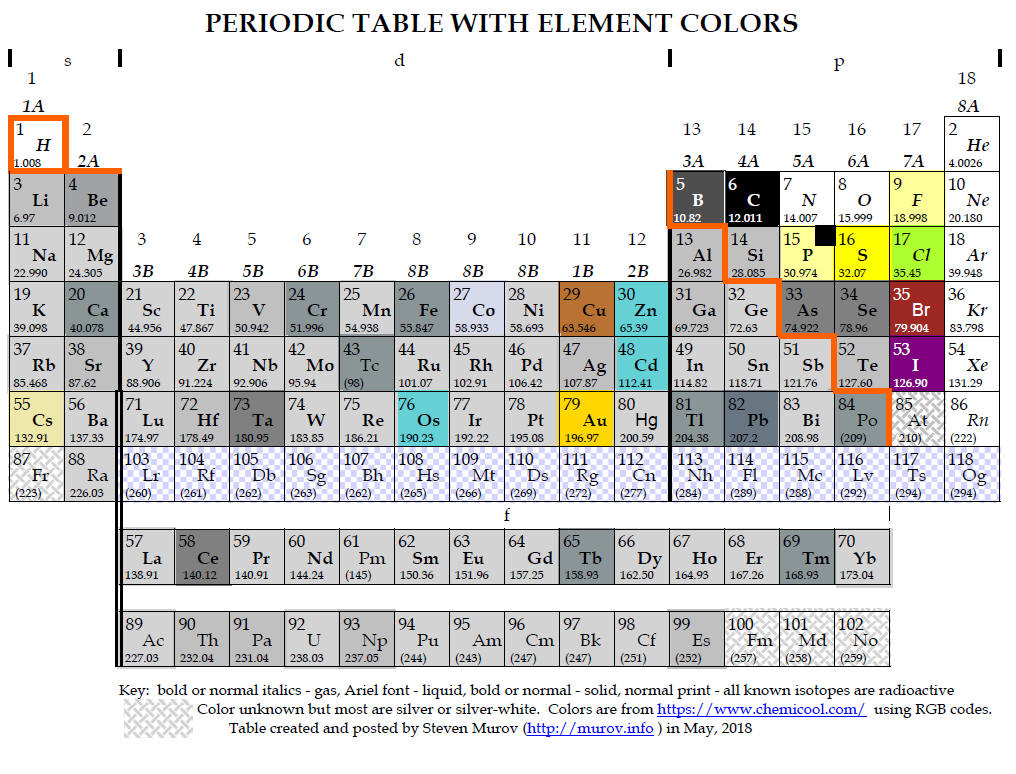
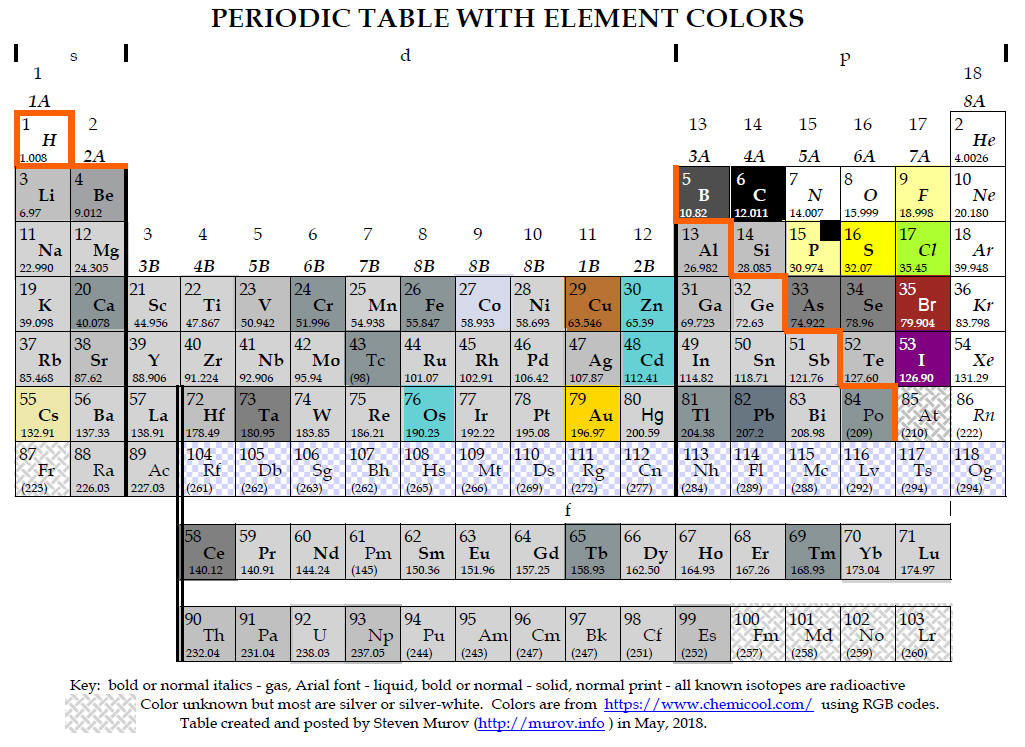
The tables above have been designed to contain elements colored to resemble the actual colors of the elements. The periodic tables with element colors can also be found at several web sites:
http://murov.info/timelines.htm
http://murov.info/periodictables.htm
http://murov.info/pertabcol1.pdf
http://murov.info/pertabcol2.pdf
http://murov.info/timeline.pptx
Please send comments and suggestions to Steve Murov, murovs@yosemite.edu