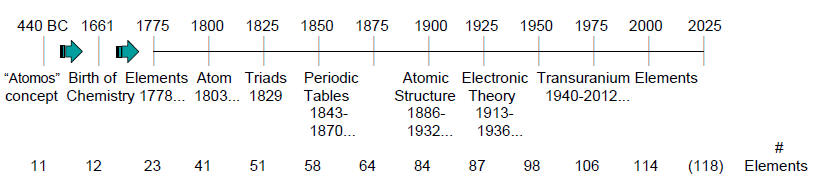
Periodic Table Timelines:
A chronology
of the events that have resulted in our present periodic table of the elements
and
a celebration of the 150th
anniversary of the Mendeleev (birthday, 02/08/1834) periodic table (1869).
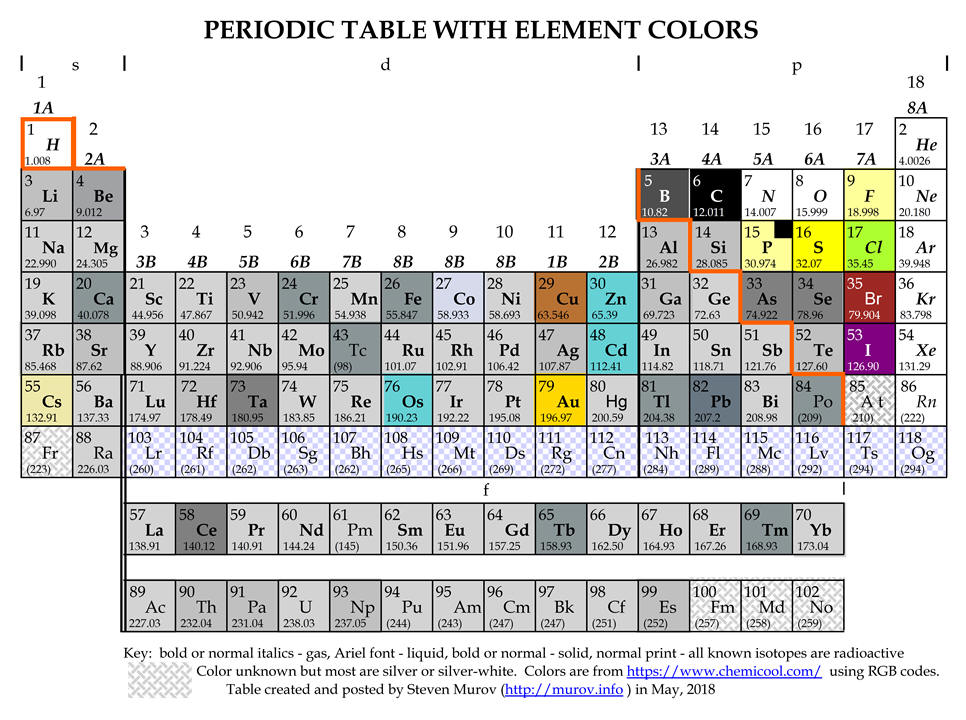
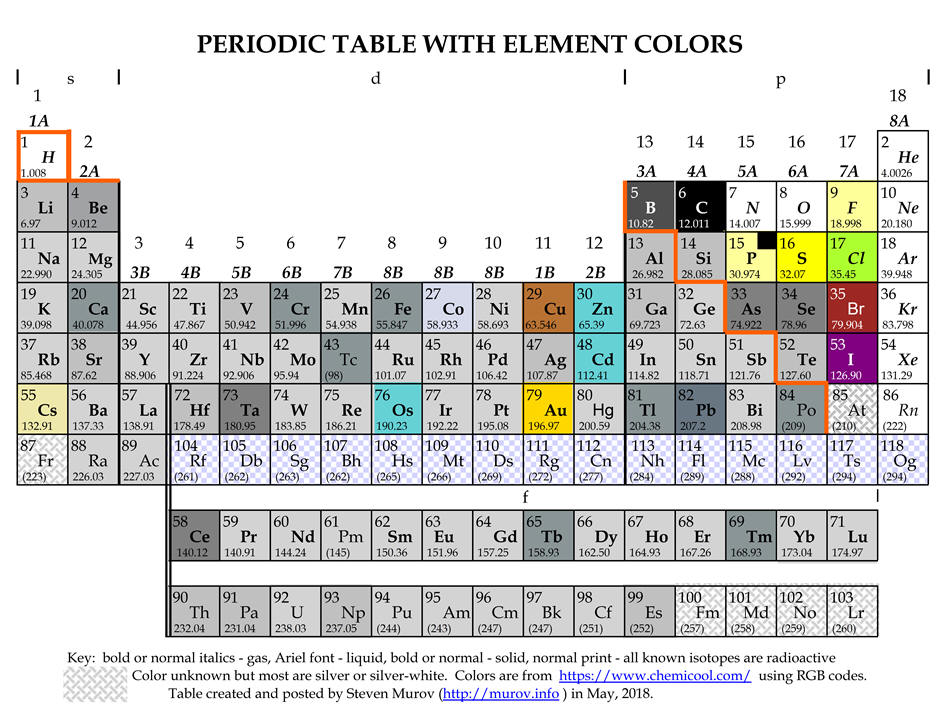
(Note: A
Powerpoint presentation of the historical developments that have culminated in
our current periodic table is available at:
http://murov.info/timeline.pptx ).
This slide presentation contains images but minimal narrative which needs to be
provided by the presenter.)
One example of a YouTube presentation on the development of the periodic
table is available at:
https://www.youtube.com/watch?v=I5H1SeepnaU
A web site containing many of the important milestones in organic chemistry is available (6/10/18) at: http://murov.info/organicmilestones.htm
https://cen.acs.org/articles/96/i1/Periodic-table-turns-150-2019.html
https://en.unesco.org/news/2019-proclaimed-international-year-periodic-table-chemical-elements
https://iupac.org/united-nations-proclaims-international-year-periodic-table-chemical-elements/
Also see:
http://www.chem.unt.edu/~jimm/REDISCOVERY%207-09-2018/

CONTENTS
Medium-long forms of the periodic table with element colors.
Selected periodic properties of the elements (ionization energy, atomic radius, valence).
Some periodic table questions and unresolved issues.
|
Image1 |
contributor |
contribution |
year |
element |
Location |
#3 |
At. # |
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
>9000 BC |
Copper |
Mid-East |
1 |
29 |
|||
|
|
|
|
7000 BC |
Lead |
Egypt |
2 |
82 |
|||
|
|
|
|
>6000 BC |
Gold |
Egypt |
3 |
79 |
|||
|
|
|
|
>5000 BC |
Silver |
Greece |
4 |
47 |
|||
|
|
|
|
>5000 BC |
Iron |
Egypt |
5 |
26 |
|||
|
|
|
|
3750 BC |
Carbon |
Egypt |
6 |
6 |
|||
|
|
|
|
3500 BC |
Tin |
Egypt |
7 |
50 |
|||
|
|
|
|
>2000 BC |
Sulfur |
China |
8 |
16 |
|||
|
|
|
|
>2000 BC |
Mercury |
China |
9 |
80 |
|||
|
|
|
|
>1600 BC |
Antimony |
|
10 |
51 |
|||
|
|
|
|
>1000 BC |
Zinc |
India |
11 |
30 |
|||
|
Empedocles |
Proposed 4 element concept – everything
derivable from earth, air, fire and water. |
450 BC |
|
Greece |
|
|
||||
|
|
Democritus,
Leucippus |
Based on reason, as contrasted with experiment, proposed that there is a
limit to the subdivision of matter that culminates in an indivisible particle called atomos. |
440 BC |
|
Greece |
|
|
|||
|
|
Plato, Aristotle |
Added a fifth element, "aether", promoted concept of continuity of
matter and
rejected concept of atoms.
As a result, atoms were not generally accepted as reality for the next
2000 years. |
340 BC |
|
Greece |
|
|
|||
| 300 | Arsenic | Egypt | 12 | 33 | ||||||
|
Jabir ibn Hayyan |
Experimentalist and alchemist recognized
by some as father of chemistry.
He developed classifications of metals and non-metals. |
800 |
|
Iran, Iraq |
|
|
||||
|
|
Robert Boyle
|
Considered by many as the first modern chemist. Author of Sceptical Chymist. |
1661 |
|
Ireland, England |
|
|
|||
|
|
Hennig Brand |
|
1669 |
Phosphorus |
Germany |
13 |
15 |
|||
|
|
Georg Ernst Stahl |
Stahl promoted the concept of phlogiston
first proposed by his mentor, Johann Joachim Becher, and then expanded
by Stahl’s student, J. H. Pott but debunked years later by Lavoisier and
others. |
1703 |
|
Germany |
|
|
|||
|
|
George Brandt |
|
1735 |
Cobalt |
Sweden |
14 |
27 |
|||
|
|
A. de Ulloa |
|
1735 |
Platinum |
Columbia |
15 |
78 |
|||
|
|
A. Cronstedt |
|
1751 |
Nickel |
Sweden |
16 |
28 |
|||
|
|
C. Younger |
|
1753 |
Bismuth |
France |
17 |
83 |
|||
|
|
Joseph Black |
|
1755 |
Magnesium |
Scotland |
18 |
12 |
|||
|
|
H. Cavendish |
|
1766 |
Hydrogen |
England |
19 |
1 |
|||
|
|
Carl Scheele,
Joseph Priestley |
|
1772 |
Oxygen |
Sweden
England |
20 |
8 |
|||
|
|
D. Rutherford |
|
1772 |
Nitrogen |
Scotland |
21 |
7 |
|||
|
|
Carl Scheele |
|
1774 |
Chlorine |
Sweden |
22 |
17 |
|||
|
|
Johan G. Gahn |
|
1774 |
Manganese |
Sweden |
23 |
25 |
|||
|
|
Antoine Lavoisier |
Considered to be founder of modern chemistry, demonstrated conservation of mass in chemical reactions within measureable limits, dealt phlogiston concept a blow and attempted to organize matter by properties. Provided empirical evidence for elements as contrasted to earlier abstract concept.
http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=3 |
1778 |
|
France |
|
|
|||
|
|
Carl Scheele |
|
1778 |
Molybdenum |
Sweden |
24 |
42 |
|||
|
|
J. & F. Elhuyar |
|
1783 |
Tungsten |
Spain |
25 |
74 |
|||
|
|
F.
Reichenstein |
|
1783 |
Tellurium |
Romania |
26 |
52 |
|||
|
|
M. H. Klaproth |
|
1789 |
Zirconium |
Germany |
27 |
40 |
|||
|
|
M. H. Klaproth |
|
1789 |
Uranium |
Germany |
28 |
92 |
|||
|
|
A. Crawford,
W. Cruickshank |
|
1790 |
Strontium |
Scotland |
29 |
38 |
|||
|
|
William Gregor |
|
1791 |
Titanium |
England |
30 |
22 |
|||
|
|
Johann Gadolin |
|
1794 |
Yttrium |
Finland |
31 |
39 |
|||
|
|
Jeremias B. Richter |
Suggested that chemical reactions have a
mathematical relationship that he called stoichiometry and proposed law
of definite proportions. |
1794 |
|
Germany |
|
|
|||
|
Joseph Proust |
Responsible for law of constant
composition. |
1794 |
|
France |
|
|
||||
|
|
L-N.
Vauquelin |
|
1797 |
Chromium |
France |
32 |
24 |
|||
|
|
L-N.
Vauquelin |
|
1798 |
Beryllium |
France |
33 |
4 |
|||
|
|
A. M.
Del Rio |
|
1801 |
Vanadium |
Spain |
34 |
23 |
|||
|
|
C. Hatchett |
|
1801 |
Niobium |
England |
35 |
41 |
|||
|
|
A. G. Ekenberg |
|
1802 |
Tantalum |
Sweden |
36 |
73 |
|||
|
|
W.
Wollaston |
|
1803 |
Palladium |
England |
37 |
46 |
|||
|
|
J. J. Berzelius,
W. v. Hisinger,
M. H. Klaproth |
|
1803 |
Cerium |
Sweden
Germany |
38 |
58 |
|||
|
|
S. Tennant |
|
1803 |
Osmium |
England |
39 |
76 |
|||
|
|
S. Tennant |
|
1803 |
Iridium |
England |
40 |
77 |
|||
|
|
W. H.
Wollaston |
|
1803 |
Rhodium |
England |
41 |
45 |
|||
|
 |
John Dalton |
Revived, utilizing experimental evidence
and work of Richter and Proust, the concept of atomos with the Dalton
atomic theory. Proposed law
of multiple proportions and developed ranking of atomic masses (actually
equivalent masses). |
1803 |
|
England |
|
|
|||
|
Joseph Louis Gay-Lussac, Alexander von Humboldt,
H. Cavendish |
Gases at constant T and P combine in
simple numerical proportions by volume, and the resulting product gases
also bear a simple proportion by volume to the volumes of the reactants. |
1808 |
|
France |
|
|
||||
|
|
Sir H. Davy |
|
1807 |
Potassium |
England |
42 |
19 |
|||
|
|
Sir H. Davy |
|
1807 |
Sodium |
England |
43 |
11 |
|||
|
|
Sir H. Davy |
|
1808 |
Calcium |
England |
44 |
20 |
|||
|
|
L. Gay-Lussac,
L-J. Thénard,
Sir H. Davy |
|
1808 |
Boron |
France,
England |
45 |
5 |
|||
|
|
Sir H. Davy |
|
1808 |
Barium |
England |
46 |
56 |
|||
|
|
Barnard Courtois |
|
1811 |
Iodine |
France |
47 |
53 |
|||
|
|
Amedeo Avogadro |
Developed law that equal volumes of
different gases contain the same number of molecules and the diatomic
nature of many gases which many years later enabled chemists (~1860) to
distinguish between equivalent and atomic masses. |
1811 |
|
Italy |
|
|
|||
|
|
William Prout
|
Proposed concept that atomic weights of
elements are whole-number multiples of the atomic weight of hydrogen
suggesting that all elements are composed of hydrogen atoms.
|
1815 |
|
England |
|
|
|||
|
|
J. A. Arfvedson |
|
1817 |
Lithium |
Sweden |
48 |
3 |
|||
|
|
J. J. Berzelius |
|
1817 |
Selenium |
Sweden |
49 |
34 |
|||
|
|
F. Stromeyer |
|
1817 |
Cadmium |
Germany |
50 |
48 |
|||
|
|
J. J. Berzelius |
|
1824 |
Silicon |
Sweden |
51 |
14 |
|||
|
|
H. C. Oersted |
|
1825 |
Aluminum |
Denmark |
52 |
13 |
|||
|
|
Antoine Balard |
|
1826 |
Bromine |
France |
53 |
35 |
|||
|
|
Jöns Jacob. Berzelius |
In addition to discovering elements,
Berzelius published list of atomic weights (later vastly improved by
Cannizzaro - 1860) and developed element symbols. |
1828 |
Thorium |
Sweden |
54 |
90 |
|||
|
|
Johann W.
Döbereiner |
Demonstrated concept of triads, groups
of elements (later realized to be in the same group) in which the mass
of the middle element was close to the average of the masses of the
first and third element. |
1829 |
|
Germany |
|
|
|||
| C. Mosander | 1839 | Lanthanum | Sweden | 55 | 57 | |||||
 |
Leopold Gmelin |
Using the concept of triads, developed a
table of 55 elements in a periodic system that contained many of the
important relationships of our modern periodic table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=268 |
1843 | Germany | ||||||
| C. Mosander | 1843 | Erbium | Sweden | 56 | 68 | |||||
|
|
C. Mosander |
|
1843 |
Terbium |
Sweden |
57 |
65 |
|||
|
|
Karl K. Klaus |
|
1844 |
Ruthenium |
Russia |
58 |
44 |
|||
|
|
Peter Kremers |
Extended the concept of triads from
vertical to both vertical and horizontal relationships. |
1856 |
|
Germany |
|
|
|||
|
|
R. W. Bunsen, G. R. Kirchhof |
|
1860 |
Cesium |
Germany |
59 |
55 |
|||
|
|
Stanislao Cannizzaro
|
Played important role in establishing
useful table of atomic masses that enabled development within a decade
of several periodic tables. |
1860 |
|
Italy |
|
|
|||
|
|
R. W. Bunsen, G. R. Kirchhof |
|
1861 |
Rubidium |
Germany |
60 |
37 |
|||
|
|
W. Crookes |
|
1861 |
Thallium |
England |
61 |
81 |
|||
|
|
Alexandre-Émile Béguyer de Chancourtois |
Ordered elements according to increasing
atomic mass in a chart that demonstrated periodic properties of the
elements.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=7
|
1862 |
|
France |
|
|
|||
|
|
F. Reich,
H. T. Richter |
|
1863 |
Indium |
Germany |
62 |
49 |
|||
|
 |
John Newlands |
Ordered elements according to increasing
atomic mass in a chart that demonstrated periodic properties of the
elements and added rule of octaves.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=8 |
1864 |
|
England |
|
|
|||
|
|
William Odling |
Ordered elements according to increasing
atomic mass in a chart that demonstrated periodic properties of the
elements.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=91 |
1864 |
|
England |
|
|
|||
|
|
Gustavus Hinrichs |
Ordered elements according to increasing
atomic mass in a spiral chart that demonstrated periodic properties of
the elements. Used spectral
evidence.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=270 |
1867 |
|
Denmark, U.S. |
|
|
|||
|
|
Jules Janssen |
|
1868 |
Helium |
France |
63 |
2 |
|||

|
Dimitri Mendeleev |
Shares with Meyer recognition for
predecessor of modern periodic table with elements ordered by atomic
mass and groups determined by chemical properties.
Te and I placed correctly despite inverted masses.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=9 |
1868,
1869 |
|
Russia |
|
|
|||

|
Lothar Meyer |
Shares with Mendeleev recognition for
predecessor of modern periodic table with elements ordered by atomic
mass and groups determined by chemical properties.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=12 |
1868,
1870 |
|
Germany |
|
|
|||
|
|
P.
Boisbaudran |
|
1875 |
Gallium |
France |
64 |
31 |
|||
|
|
J. C. G.
Marignac |
|
1878 |
Ytterbium |
Switzerland |
65 |
70 |
|||
|
|
Per T. Cleve |
|
1878 |
Holmium |
Sweden |
66 |
67 |
|||
|
|
Lars F. Nilson |
|
1879 |
Scandium |
Sweden |
67 |
21 |
|||
|
|
Per T. Cleve |
|
1879 |
Thulium |
Sweden |
68 |
69 |
|||
|
|
P.
Boisbaudran |
|
1879 |
Samarium |
France |
69 |
62 |
|||
|
|
J. C. G.
Marignac |
|
1880 |
Gadolinium |
Switzerland |
70 |
64 |
|||
|
|
C. F. A. Welsbach |
|
1885 |
Praseodymium |
Germany |
71 |
59 |
|||
|
|
C. F. A. Welsbach |
|
1885 |
Neodymium |
Austria |
72 |
60 |
|||
|
|
C. Winkler |
|
1886 |
Germanium |
Germany |
73 |
32 |
|||
|
|
P.
Boisbaudran |
|
1886 |
Dysprosium |
France |
74 |
66 |
|||
|
|
Henri Moissan |
|
1886 |
Fluorine |
France |
75 |
9 |
|||
|
|
William Crookes |
Developed Crookes tube (1975) enabling
discovery of electron and also suggested atomic weights are an average
of different kinds of atoms of the same element (concept of isotopes). |
1886 |
|
England |
|
|
|||
|
|
Eugen Goldstein |
Discovered rays that were eventually
realized to be hydrogen nuclei or protons. |
1886 |
|
Germany |
|
|
|||
|
|
W. Ramsay,
Lord Rayleigh |
|
1894 |
Argon |
England,
Scotland |
76 |
18 |
|||
|
|
W. Röntgen |
Discovered X-rays. |
1895 |
|
Germany |
|
|
|||
|
|
H. Becquerel |
Discovered radioactivity that enabled
Marie Curie and others to isolate radioactive elements that filled in
gaps in periodic table and for Rutherford to design gold foil expt. |
1896 |
|
France |
|
|
|||
|
|
|
|
1896 |
Europium |
France |
77 |
63 |
|||
|
J. J. Thomson |
Discovered electron using Crookes’ tube
and demonstrated that this tiny negatively charged particle had a huge
charge to mass ratio (e/m) and was present in atoms of all elements.
Also found evidence for protons.
In 1904, he proposed “plum pudding” model for atom that was
disproved by Rutherford in 1911. |
1897 |
|
|
|
|
||||
|
|
W. Ramsay, M. M. Travers |
|
1898 |
Krypton |
Scotland |
78 |
36 |
|||
|
|
W. Ramsay, M. M. Travers |
|
1898 |
Neon |
Scotland |
79 |
10 |
|||
|
|
W. Ramsay, M. M. Travers |
|
1898 |
Xenon |
Scotland |
80 |
54 |
|||
|
|
Marie Curie,
Pierre Curie |
With her husband Pierre, discovered polonium and later other radioactive elements.
|
1898 |
Polonium |
France |
81 |
84 |
|||
|
|
M. & P. Curie |
|
1898 |
Radium |
France |
82 |
88 |
|||
|
|
A-L. Debierne |
|
1899 |
Actinium |
France |
83 |
89 |
|||
|
F. E. Dorn |
|
1900 |
Radon |
Germany |
84 |
86 |
||||
|
|
Max Planck |
Began modern era of quantum mechanics
when he showed that energy did not flow in a steady continuum, but was
delivered in discrete packets called quanta with E = hn. |
1900 |
|
Germany |
|
|
|||

|
William Ramsay |
Discovery of inert gases in late 1890’s
(helium observed in sun in 1868 but isolated by William Ramsay and
Raleigh in 1895) by Ramsay and co-workers led Ramsay, Mendeleev and
others to add inert gases to periodic table.
See:
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All
|
1902 |
|
Scotland |
|
|
|||
|
|
Hantaro Nagaoka |
Proposed solar system like model of the
atom but not accepted until gold foil experiment of Rutherford in 1911. |
1904 |
|
Japan |
|
|
|||
|
|
Richard Abegg |
Developed concept of valence and
explained inertness of noble gases based on octave of electrons in outer
shell |
1904 |
|
Germany |
|
|
|||
|
|
Alfred Werner |
Presented periodic table with
substantial resemblance to most popular form used today.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=64
|
1905 |
|
Switzerland |
|
|
|||
|
|
Albert Einstein |
Light which had been considered a form
of electromagnetic waves, must also be thought of as particle-like.
Theories of relativity also are important considerations in
electron orbitals of high atomic number. |
1905 |
|
Germany |
|
|
|||
|
|
G. Urbain,
C. F. A. Welsbach Charles James |
|
1907 |
Lutetium |
France, Austria, U.S. |
85 |
71 |
|||

|
Jean Baptiste Perrin |
Strongly supported atomic theory, laid
groundwork for Thomson’s discovery of the electron and proposed solar
system model for atom (not accepted until 1911). |
1907 |
|
France |
|
|
|||
|
|
Antonius Johannes van den Broek |
First to change ordering of elements
from atomic mass to charge in the nucleus (later called atomic number). |
1907 |
|
Netherlands |
|
|
|||
|
|
Robert Millikan |
Oil drop experiment determined charge on
electron and enabled calculation of mass of electron from Thomson’s e/m
ratio. |
1909 |
|
United States |
|
|
|||
|
|
Ernest Rutherford |
Performed gold foil experiment that
demonstrated nuclear model for atom with tiny nucleus but with almost
all the mass of the atom. Before
gold foil experiment, he showed that alpha particles are helium nucleii.
Also, often given credit for discovery of proton (however, see Goldstein
in 1886) |
1911 |
|
England
(born in New Zealand) |
|
|
|||
|
|
Frederick Soddy |
Worked with Rutherford and elevated Crooke’s
suggestion of isotopes into a theory.
In 1913, Hevesy and Paneth provided evidence that isotopes
chemically behave the same. |
1912 |
|
England |
|
|
|||
|
|
Henry Moseley |
Using X-rays, demonstrated that the
number of protons (atomic number) as suggested by van den Broek instead
of atomic mass is the correct basis for the ordering of the elements. |
1913 |
|
England |
|
|
|||

|
Niels Bohr |
Applied quantum theory to atoms. Using a planetary model, derived a correct mathematical description of electron in hydrogen but model failed for multiple electron atoms. Proposed Aufbau Principle. Attempted to use electron structure to explain shape of periodic table. Produced symmetrical periodic table that improved tables of T. Bayley and J. Thomsen and was improved again by E. Scerri in 1997. https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=19 |
1913,
1922 |
|
Denmark |
|
|
|||
|
|
K. Fajans,
Otto Göhring |
|
1913 |
Protactinium |
Germany |
86 |
91 |
|||
|
|
Gilbert Lewis |
Developed concept of electron pairs,
bonding and Lewis structures for atoms and molecules.
Expanded work on valence concepts of Richard Abegg. |
1916 |
|
U.S. (CA) |
|
|
|||
|
|
I. Langmuir, C. Bury,
J. M. Smith |
Arranged elements according to electron
arrangement.
|
1919,
1921,
1924 |
|
U.S.,
England |
|
|
|||
|
|
Francis Aston |
Invented mass spectrometer and using it,
was the first to experimentally demonstrate existence of isotopes of
many elements. |
1922 |
|
England |
|
|
|||
|
|
G. C. Hevesy,
Dirk Coster |
|
1923 |
Hafnium |
Denmark |
87 |
72 |
|||

|
Edmund Stoner |
Improved Bohr’s attempt to correlate
periodic table with electron theory by adding a third quantum number. |
1924 |
|
England |
|
|
|||
|
|
Louis-Victor de Broglie |
Predicted wave nature of electrons and
all matter. |
1924 |
|
France |
|
|
|||
|
|
Wolfgang Pauli |
Added spin quantum number to make 4
quantum numbers that enable strong correlation of electron orbital
theory to experimental properties and shape of periodic table.
Introduced Pauli Exclusion Principle. |
1924 |
|
Switzerland |
|
|
|||
|
|
Werner Heisenberg |
Used matrix mechanics to mathematically
describe electron orbitals.
His results were later shown to give results equivalent to
Schrödinger’s wave equations.
Known for important Heisenberg Uncertainty Principle. |
1925 |
|
Germany |
|
|
|||
|
|
Erwin Schrödinger |
Using concept of duality of matter,
developed wave equations that, in theory, enable correct calculations of
electron orbitals and properties.
Practically speaking, solving the equations requires simplifying
assumptions. |
1926 |
|
Austria |
|
|
|||
|
|
W. & I. Noddack,
Otto Berg |
|
1925 |
Rhenium |
Germany |
88 |
75 |
|||
|
|
Friedrich Hund |
Produced Hund’s rule which enhance
application of Bohr’s Aufbau Principle. |
1927 |
|
Germany |
|
|
|||
|
|
Paul Dirac |
Formulated relativistic form of quantum
mechanics that described energy levels of electrons. |
1928 |
|
England |
|
|
|||
|
|
Charles Janet |
Developed left step periodic table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=152
|
1928 |
|
France |
|
|
|||

|
James Chadwick |
Confirmed existence of and determined
the mass of the neutron. |
1932 |
|
England |
|
|
|||
|
|
Erwin Madelung |
Madelung’s rule based on Janet’s
suggestions gives order of filling of electron orbitals.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=777 |
1936 |
|
Germany |
|
|
|||
|
|
C. Perrier,
E. G. Segre |
|
1937 |
Technetium |
Italy |
89 |
43 |
|||
| Otto Hahn and Fritz Strassmann discovered nuclear fission that was explained and predicted by Lise Meitner. |
1937, 1938 |
Germany | ||||||||
| Hans Bethe | Discovered nuclear fusion in the sun. | 1938 | U.S. (N.Y.) | |||||||
|
|
M. Perey |
|
1939 |
Francium |
France |
90 |
87 |
|||
|
|
D. R. Corson, K.R. Mackenzie, Emilio
Segré |
|
1940 |
Astatine |
U.S. (CA) |
91 |
85 |
|||
|
|
E. McMillan,
P. H. Abelson |
|
1940 |
Neptunium |
U.S. (CA) |
92 |
93 |
|||
|
|
Glenn Seaborg, E. McMillan,
J. W.
Kennedy, Arthur Wahl |
|
1940 |
Plutonium |
U.S. (CA) |
93 |
94 |
|||
|
|
Glenn Seaborg, Ralph James, Albert
Ghiorso |
|
1944 |
Curium |
U.S. (CA) |
94 |
96 |
|||
|
|
Glenn Seaborg, Ralph A. James, L. O.
Morgan Albert Ghiorso |
|
1944 |
Americium |
U.S. (CA) |
95 |
95 |
|||
|
|
J. .A. Marinsky,
L. E. Glendenin, C. D. Coryell |
|
1945 |
Promethium |
U.S. (TN) |
96 |
61 |
|||
|
|
Emilio Gino Segrè |
Produced chart of isotopes arranged by increasing atomic number.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=231 |
1945 |
|
Italy, U.S. |
|
|
|||
|
|
Glenn Seaborg |
In addition to leading the group that used transmutation to synthesize many transuranium elements, produced most complete version of modern periodic table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=522 |
1945 |
|
U.S. (CA) |
|
|
|||
|
|
S. Thompson, Albert Ghiorso, Glenn
Seaborg. |
|
1949 |
Berkelium |
U.S. (CA) |
97 |
97 |
|||
|
|
S. Thompson, Kenneth Street, Albert
Ghiorso Glenn Seaborg |
|
1950 |
Californium |
U.S. (CA) |
98 |
98 |
|||
|
|
Albert Ghiorso |
|
1952 |
Einsteinium |
U.S. |
99 |
99 |
|||
|
|
Albert Ghiorso |
|
1952 |
Fermium |
U.S. (CA) |
100 |
100 |
|||
|
|
Albert Ghiorso, Bernard Harvey, Gregory
Choppin, S. Thompson, Glenn
Seaborg |
|
1955 |
Mendelevium |
U.S. (CA) |
101 |
101 |
|||
|
|
Albert Ghiorso, T. Sikkeland,
Almon E. Larsh R. M. Latimer |
|
1961 |
Lawrencium |
U.S. (CA) |
102 |
103 |
|||
|
|
Maria G. Mayer,
Hans Jensen |
Developed nuclear shell model that
contributes to understanding of nuclear stability.
|
1963 |
|
U.S.,
Germany |
|
|
|||
|
|
M. Gell-Mann,
G. Zweig |
Proposed quark model.
Although not needed to explain the periodic table, quarks enhance
our understanding of the nucleus.
|
1964 |
|
|
|
|
|||
|
|
Georgy Flerov |
|
1964 |
Rutherfordium |
USSR |
103 |
104 |
|||
|
|
Georgy Flerov |
|
1966 |
Nobelium |
USSR |
104 |
102 |
|||
|
|
disputed |
|
1967 |
Dubnium |
USSR or U.S. |
105 |
105 |
|||
|
|
Albert Ghiorso,
Y. Oganessian |
|
1974 |
Seaborgium |
U.S. (CA) or Russia |
106 |
106 |
|||
|
|
P. Armbruster, G. Münzenber |
|
1981 |
Bohrium |
Germany |
107 |
107 |
|||
|
|
P. Armbruster, G. Münzenber |
|
1982 |
Meitnerium |
Germany |
108 |
109 |
|||
|
|
P. Armbruster, G. Münzenber |
|
1984 |
Hassium |
Germany |
109 |
108 |
|||
|
|
S. Hofmann,
P. Armbruster, G.
Münzenber |
|
1994 |
Darmstadtium |
Germany |
110 |
110 |
|||
|
|
P. Armbruster, G. Münzenber |
|
1994 |
Roentgenium |
Germany |
111 |
111 |
|||
|
|
S.Hofmann, Victor Ninov |
|
1996 |
Copernicium |
Germany |
112 |
112 |
|||
|
|
Y. Oganessian |
|
1998 |
Flerovium |
Russia |
113 |
114 |
|||
|
|
Y. Oganessian Ken Moody |
|
2000 |
Livermorium |
Russia,U.S. |
114 |
116 |
|||
|
|
Y. Oganessian Ken Moody |
|
2003 |
Moscovium |
Russia, U.S. |
115 |
115 |
|||
|
|
Y. Oganessian |
|
2006 |
Oganesson |
Russia |
116 |
118 |
|||
|
|
Y. Oganessian |
|
2010 |
Tennessine |
Russia, U.S. |
117 |
117 |
|||
|
|
Kosuke Morita |
|
2012
|
Nihonium |
Japan |
118 |
113 |
|||
|
|
Mark Winter |
Produces continuously updated website
devoted to the Periodic Table.
https://www.webelements.com/ |
1993 - current |
|
England |
|
|
|||
|
|
Eric R. Scerri |
The Periodic Table: Its Story
and Its Significance,
Oxford
University Press, N.Y. A
history of the development of the periodic table.
|
2007 |
|
U.S. |
|
|
|||
|
|
Mark R. Leach |
Updates hot links to most periodic table
websites and element discoveries.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All |
2003- current |
|
England |
|
|
|||
 |
Theodore Gray |
http://periodictable.com/index.html For an extensive list of periodic properties with graphing capabilities, visit: http://periodictable.com/Properties/A/CrustAbundance.htm |
current | U.S. | ||||||
1Images of most contributors of concepts included but not discoverers of elements with the exceptions of Marie Curie and William Ramsay
2RGB codes used for colors of elements taken from Chemicool. Color is unknown but suspected to be silvery for elements with dark blue font.
3Number
of known elements.

|
Year |
#1 |
Concept of the atom |
Periodic table based on properties |
Theory of Periodic Table |
|
|
|
|
|
|
|
440 BC |
11 |
Democritus, Leucippus suggested that
matter is made up of indivisible particles called “atomos”.
|
|
|
|
340 BC |
11 |
Aristotle, Plato reject atomos concept
and claim matter is continuous.
Continuity of matter concept dominates for 2100 years until
evidence emerges in support of the atomic theory (1803). |
|
|
|
1778 |
23 |
|
Antoine. Lavoisier demonstrates conservation of mass (within detectable limits) in chemical reactions and prepares list of 33 substances including 23 elements arranged according to properties (gases, non-metals, metals, earths)
http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=3 |
|
|
1794 |
31 |
Jeremias B. Richter and Joseph Proust
discovered laws of definite proportions and constant composition. |
|
|
|
1803 |
41 |
Results of Richter and Proust are inconsistent with continuity concept of matter but explainable using
atomos concept. John Dalton
introduced empirically based atomic theory.
1.
Elements are made of extremely small particles called atoms.
2.
Atoms of a given element are the same in size, mass and other
properties; atoms of different elements differ in size, mass and other
properties.
3.
Atoms cannot be subdivided, created or destroyed.
4.
Atoms of different elements combine in simple whole-number ratios
to form chemical compounds.
5.
In chemical reactions, atoms are combined, separated or
rearranged. |
|
|
|
1815 |
47 |
|
William Prout proposed concept that
atomic weights of elements are whole-number multiples of the atomic
weight of hydrogen suggesting that all elements are composed of hydrogen
atoms. |
|
|
1829 |
54 |
|
Johann W.
Döbereiner demonstrated concept of triads, groups of elements
(later realized to be in the same group) in which the mass of the middle
element was close to the average of the masses of the first and third
element. |
|
|
1843 |
55 |
|
Leopold Gmelin using the concept of
triads, developed a table of 55 elements in a periodic system that
contained many of the important relationships of our modern periodic
table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=268
|
|
|
1860 |
59 |
|
Stanislao Cannizzaro played important
role in establishing useful table of atomic masses that enabled
development within a decade of several periodic tables. |
|
|
1862-1867 |
62 |
|
A.-É. Béguyer de Chancourtois,
F. Reich, H. T. Richter, John Newlands,
William Odling, Gustavus Hinrichs
ordered elements according to increasing atomic mass in charts that
demonstrated periodic properties of the elements.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All |
|
|
1868- 1870 |
63 |
|
Dimitri Mendeleev and independently
Lothar Meyer published predecessors of modern periodic table with
elements ordered by atomic mass and groups determined by chemical
properties.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=9
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=12 |
|
|
1886 |
75 |
William Crookes suggested atomic weights
are an average of different kinds of atoms of the same element (concept
of isotopes). Crookes in 1875 also developed Crookes’ tube which enabled
discovery of electron (1897) |
|
|
|
1886 |
75 |
Eugen Goldstein discovered rays that
were eventually realized to be hydrogen nuclei or protons. |
|
|
|
1895 |
76 |
W. Röntgen discovered X-rays.
|
|
|
|
1896 |
77 |
H. Becquerel discovered radioactivity
that enabled Marie Curie and others to isolate radioactive elements and
for Rutherford to design gold foil expt. |
|
|
|
1897 |
77 |
J. J. Thomson using Crookes’ tube
discovers electron and determines e/m ratio. |
|
|
|
1898 |
80 |
|
With her husband Pierre, Marie Curie
discovered polonium and later other radioactive elements
that filled in gaps in periodic table. |
|
|
1900 |
84 |
|
|
Max Planck initiated modern era of
quantum mechanics when he showed that energy did not flow in a steady
continuum, but was delivered in discrete packets called quanta with E =
hn. |
|
1902 |
84 |
|
William Ramsay and co-workers discovery
of inert gases in late 1890’s (helium observed in sun in 1868 but
isolated by William Ramsay and Raleigh in 1895) led Ramsay, Mendeleev
and others to add inert gases to periodic table.
See:
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All
|
|
|
1904 |
84 |
J. J. Thomson proposes “plum pudding”
model of atom. |
|
|
|
1904 |
84 |
Hantaro Nagaoka proposed solar system
like model of the atom that was not accepted until gold foil experiment
of Rutherford in 1911. In
1907, Jean Baptiste Perrin also promoted solar system model for atom. |
|
|
|
1905 |
84 |
|
Alfred Werner presented periodic table
with substantial resemblance to most popular form used today.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=64
|
Albert Einstein showed that light which
had been considered a form of electromagnetic waves, must also be
thought of as particle-like (photons). Einstein’s relativity theories
also are important considerations in electron orbitals of high atomic
number. |
|
1907 |
85 |
|
Antonius Johannes van den Broek was the
first to change ordering of elements from atomic mass to nuclear charge
(later called atomic number). |
|
|
1908 |
85 |
Robert Millikan’s
oil drop experiment determined charge on electron and enabled
calculation of mass of electron from Thomson’s e/m ratio. |
|
|
|
1911 |
85 |
Ernest Rutherford performed gold foil
experiment that demonstrated nuclear model for atom with tiny nucleus
with almost all of the mass of the atom.
Before gold foil experiment, he showed that alpha particles are helium
nucleii.
Also often given credit for discovery of proton (however, see
Goldstein in 1886) |
|
|
|
1912 |
85 |
Frederick Soddy worked with Rutherford and elevated Crooke’s suggestion (1886) of isotopes into a theory. In 1913,
Hevesy and Paneth provided evidence that isotopes chemically behave the
same. |
|
|
|
1913 |
85 |
Henry Moseley using X-rays, demonstrated
that the number of protons (atomic number) as suggested by van den Broek
instead of atomic mass is the correct basis for the ordering of the
elements. |
|
Niels Bohr applied quantum theory to
atoms. Using a planetary
model, derived a correct mathematical description of the electron in
hydrogen but model failed for multiple electron atoms.
Proposed Aufbau Principle.
Attempted to use electron structure with two quantum numbers (n,
l) to explain shape of periodic
table. |
|
1922 |
86 |
Francis Aston invented mass spectrometer
and using it, was the first to experimentally demonstrate existence of
isotopes of many elements. |
Niels Bohr produced symmetrical periodic table that improved tables of T. Bayley and J. J. Thomson and was improved again by E. Scerri in 1997. https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=19 |
|
|
1924 |
87 |
|
|
Edmund Stoner improved Bohr’s attempt to
correlate periodic table with electron theory by adding a third quantum
number (n,
l, m). |
|
1924 |
87 |
|
|
Wolfgang Pauli added spin quantum number
to make 4 quantum numbers (n,
l, m, s) that enable strong
correlation of electron orbital theory with experimental properties and
shape of periodic table.
Introduced Pauli Exclusion Principle (all electrons for an atom must
have distinct set of quantum numbers). |
|
1924 |
87 |
|
|
Louis-Victor de Broglie showed wave
nature of electrons and all matter. |
|
1925, 1926 |
87 |
|
|
Werner Heisenberg and Erwin Schrödinger
using different approaches, developed wave mechanical equations for
correctly describing electronic orbitals.
Practically speaking, equations are extremely difficult to solve without
making simplifying assumptions. |
|
1927 |
88 |
|
|
Friedrich Hund produced Hund’s rule
(every orbital in a sublevel is singly occupied before any orbital is
doubly occupied) which enhanced application of Bohr’s Aufbau Principle. |
|
1928 |
88 |
|
Charles Janet developed left step
periodic table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=152
|
Paul Dirac formulated relativistic form
of quantum mechanics that described energy levels of electrons. |
|
1932 |
88 |
James Chadwick confirmed existence of
and determined the mass of the neutron. |
|
|
|
1936 |
88 |
|
|
Erwin Madelung’s rule based on Janet’s
suggestions gives order of filling of electron orbitals.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=777 |
|
1936 ... |
Nuclear reactions including transmutation, nuclear fission and nuclear
fusion are discovered and exploited. |
|||
|
1945 |
96 |
Emilio Gino Segrè
produced chart of isotopes arranged by increasing atomic number.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=231
|
Glenn Seaborg, in addition to leading
the group that used transmuation to synthesize many transuranium elements, produced
most complete version of modern periodic table.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=522
|
|
|
1963 |
102 |
Maria G. Mayer, Hans Jensen developed
nuclear shell model that contributes to understanding of nuclear
stability. |
|
|
|
1964 |
102 |
M. Gell-Mann, George Zweig proposed
quark model. Although not
needed to explain the periodic table, quarks enhance our understanding
of the nucleus. |
|
|
|
2007 |
116 |
|
Eric R. Scerri authors
The Periodic Table:
Its Story and Its Significance, Oxford University Press, N.Y.
A history of the development of the periodic table. |
|
|
1993-
2018 |
109
118 |
|
Mark Winter posted website devoted to
the Periodic Table that is continually updated.
https://www.webelements.com/ |
|
|
2003
2018 |
114
118 |
|
Mark R. Leach posted hot links to most
periodic table sites and element discoveries.
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=Al
. |
|
|
... 2018 |
118 |
Theodore Gray provides commercially available beautiful periodic tables
and website with extensive list of properties with grpahing
capibilities.
http://periodictable.com/index.html http://periodictable.com/Properties/A/CrustAbundance.html |
1images
generally only provided for conceptual contributions and not for discoverers of
elements (exceptions include Curie and Ramsay)
2number
of known elements discovered by the year indicated.
The excellent book by Eric R. Scerri (The
Periodic Table: Its Story and Its
Significance, 2007, Oxford University Press, N.Y.) and the web site of Mark
Leach
(https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All)
were the sources of much of the information
included in the timelines.
Medium-long forms of the periodic table with element colors.
A limited search for a periodic table with the colors of the elements has not yet located a table of this type. However, Theodore Gray has produced many wonderful tables including one with images of authentic samples of the elements (http://periodictable.com/) and a commercially available model that contains sample of most of the elements (http://www.periodictable.co.uk/). While the tables below are not nearly as fascinating or interesting as Gray's tables, the tables below have been designed to enable viewers to focus on the property of color. Most of the colors have been extracted from https://www.chemicool.com/ primarily because it often included a two word description of the color. Other sites referred to were Theodore Gray's http://periodictable.com/Properties/A/Color.html and Mark Winter's https://www.webelements.com/. For some elements, the colors listed on Internet sites are not always in agreement. Rene Vernon, the author of a paper on metalloids, https://pubs.acs.org/doi/pdfplus/10.1021/ed3008457 has contributed valuable comments about the colors of boron, phosphorus, iodine, cesium and astatine. Some of these color issues are because the most stable allotrope (e.g., phosphorus) is not always the most abundant allotrope.
boron - the most stable allotrope of boron is the beta rhombohedral crystalline state. Colors reported for this state range from shiny silver-grey to grey to dark to black. It is represented as dark grey below.
phosphorus - the most stable allotrope is black but the most common form is described as white to pale yellow. A very light yellow has been used below with a black insert.
iodine - while silver is sometimes mentioned, the overwhelming consensus is that iodine crystals are in the violet or purple range.
astatine - although astatine has been observed, due to its transient existence, it has apparently not been possible to determine its color. Some web sites conclude that it should have some metallic properties and as a result have a silvery color. Other web sites suggest as progression is made down group 7A (17), the color continuously darkens with a presumption that astatine should be near black. It is left in these tables as unknown like francium and the elements with atomic numbers above 99.
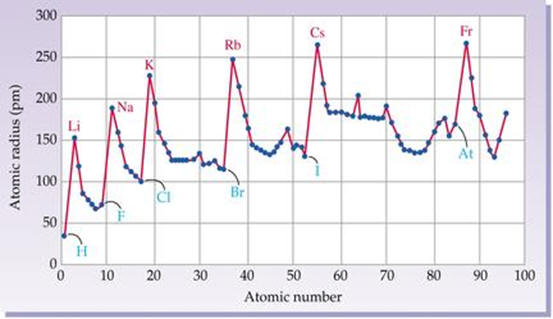
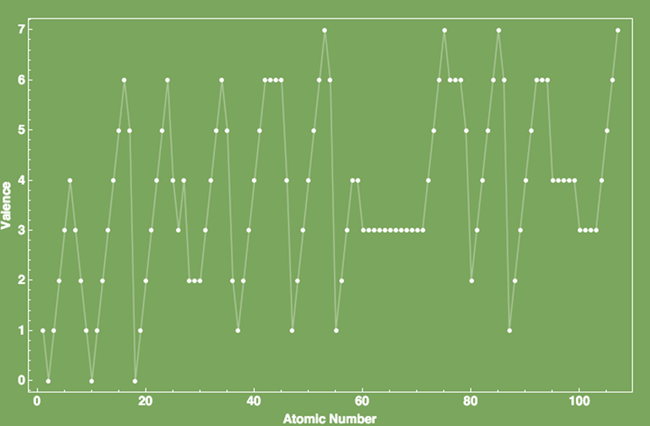
Selected periodic properties of the elements
(ionization energy, atomic radius, valence).
For an extensive list of periodic properties with graphing capabilities please
visit:
http://periodictable.com/Properties/A/CrustAbundance.html .

medium-long and long (not up-to-date with names)
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=371
(medium long and long but not up-to-date with names)
long form
https://commons.wikimedia.org/wiki/File:Periodic_table_large-long.svg
https://kaiserscience.wordpress.com/chemistry/the-periodic-table/alternative-periodic-tables/
medium-long form
https://iupac.org/what-we-do/periodic-table-of-elements/
http://murov.info/pertab-trad.pdf
left step
https://jeries.rihani.com/symmetry/index6c.html
pyramidal version (not up-to-date with names)
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=19
2.
The positions of some elements in periodic tables are still disputed.
a.
Does hydrogen belong in the alkali metals group or the halogen group or neither?
b. There
are some claims that second period elements have properties inconsistent with
the remaining members of their groups.
Explain this statement.
c.
Some medium-long periodic tables have
lanthanum part of the “f” group of
elements (split out from the periodic table), others have lutetium as a member
of the 14 elements and still others include 15 elements in the “f” group .
What is the best placement of these two elements?
Part of the issue is the priority of chemical properties versus
electronic structure. If electronic
structure is taken as the determining criteria, are there other elements that
are misplaced in the periodic table?
3.
The IUPAC numbers the groups from 1 through 18 but American periodic tables
often have A and B group elements with the numbers running from 1A through 8A
and 1B through 8B. State the
advantages and disadvantages of each and your preference (for a table with both,
see tables above or:
http://murov.info/pertab-trad.pdf.
4.
Is the periodic table universal or could there be differences on another planet?
For example, consider the universality of atomic masses.
5.
Does the periodic table contain any isotope information?
Consider use of the atomic mass as a source of isotope information (See:
S. Murov, Chem 13 News, March,
2010. “Promoting Insight:
Atomic Mass”.
6. The periodic tables above attempt to illustrate the approximate colors of the elements. The orange staircase in the two periodic tables is commonly included in many periodic tables to very qualitatively separate the metals and the non-metals. Do the colors of the elements also help to distinguish metals from non-metals and, if so, does this method correlate with the staircase model? Which method do you think has more merit? (Note: It is often suggested that the elements adjacent to the staircase are metalloids, semiconductors and/or semimetals. The consensus is that boron, silicon, germanium, arsenic, antimony and tellurium are metalloids with a few others in the questionable category. For a discussion of criteria used to characaterize metalloid properties, please see: https://pubs.acs.org/doi/pdfplus/10.1021/ed3008457 ).
7.
Calculations indicate that stability of nuclei depend on the neutron to proton
ratio and predict an island of stability above atomic number 110.
Is it possible that there are some “longer lived isotopes” with atomic
number above 110? (e.g., see:
https://en.wikipedia.org/wiki/Island_of_stabilitybeyond_118)
8.
What is the probability that elements with atomic number greater than 118 will
ever be synthesized?
(e.g., see:
https://www.chemistryworld.com/news/beyond-element-118-the-next-row-of-the-periodic-table/9400.article
9.
In some cases, discoveries have been made virtually simultaneously
by different people in different countries (e.g., 1772, 1963).
Is this just a coincidence or are other factors in play here?