 Is
Water Weird?
Is
Water Weird?
 Is
Water Weird?
Is
Water Weird?
Although oxygen and water appear to be simple substances, both are vital for
life and have behaviors that are not necessarily easy to predict.
However, a deeper dive into their structures and properties often leads
to an understanding of their apparent deviation from normalcy.
While Lewis structures are very powerful predictive tools, the structure
that results from Lewis structure guidelines for
oxygen is one of the very few Lewis structures that incorrectly depicts the electronic structure
of a substance. Use of a molecular
orbital model approach does correctly indicate that oxygen (O2)
should exist as a triplet state.
For water, the Lewis structure approach does correctly result in a model with a
bent structure. Consideration of
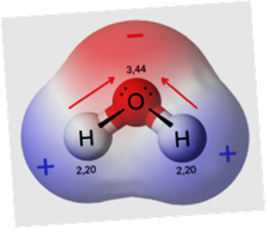
electronegativities of oxygen and hydrogen adds the
information that water should be very polar.
Using this information coupled with the added feature of hydrogen
bonding, it is possible to explain the commonly claimed anomalous behaviors of
water.
deviation from normalcy.
While Lewis structures are very powerful predictive tools, the structure
that results from Lewis structure guidelines for
oxygen is one of the very few Lewis structures that incorrectly depicts the electronic structure
of a substance. Use of a molecular
orbital model approach does correctly indicate that oxygen (O2)
should exist as a triplet state.
For water, the Lewis structure approach does correctly result in a model with a
bent structure. Consideration of
electronegativities of oxygen and hydrogen adds the
information that water should be very polar.
Using this information coupled with the added feature of hydrogen
bonding, it is possible to explain the commonly claimed anomalous behaviors of
water.

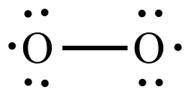

The
models of water below are consistent with the Lewis structure for water.


If
hybridization is not considered, the hydrogens should be bonded to the oxygen
via p orbitals and the bond angle would be 90o.
For H2S, this model is very good as the experimental bond
angle is 92.1o but is 14o off
for water. Because
there are four groups of electrons around the oxygen, hybridization and electron
repulsion models predict that the oxygen should be sp3 hybridized and
have 109.5 degree bond angles. This
model still has a 5 degree discrepancy.
Some might argue that the oxygen is only partially hybridized while other
arguments for the discrepancy usually suggest that since the electron pairs on
the oxygen are not involved in bonding, there is more repulsion than for bonding
electrons. The non-bonded orbitals are forced a little farther apart causing
the hydrogens to be pushed together.
For our purposes, the most important
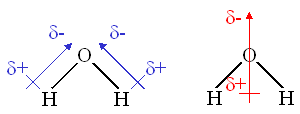 observation
is that the molecule is bent with bond dipoles having the negative terminus on
the oxygen starting from each of the hydrogens.
Part of these dipoles cancel but there is still a big net dipole directed
from midway between the hydrogens towards the oxygen.
observation
is that the molecule is bent with bond dipoles having the negative terminus on
the oxygen starting from each of the hydrogens.
Part of these dipoles cancel but there is still a big net dipole directed
from midway between the hydrogens towards the oxygen.
Molecular mass.
The molecular mass of water, 18 g/mole, is very low for a compound.
While there are several elements with low atomic mass values that are
solids at room temperature, most compounds with low molecular mass are gases at
room temperature. Water being a
liquid at room temperature makes water unusual and states of matter for H2O
will be discussed again later. The
low molecular mass value impacts many of the properties of water sometimes
leading to the claim that water behaves uniquely.
Molarity of Pure Liquid.
When we think about the range of possible molarity values, about the
highest we commonly encounter in the lab is 18 M for concentrated (98%) sulfuric
acid. 47% NaOH also is close to 18
M and 49% HF is 29 M with 90% formic acid also high at 24 M.
The values for HF and formic acid are high for the same reason that pure
water has the very high molarity of about 56 M.
Using the density of water as 1 g/cm3 means that the molarity
of water can be approximated by the following: (1000g/L)(1 mole/18 g) = 56
moles/L. The very high result is
due to division by the low molecular mass value.
For students doing problems involving the calculation of molarity, any
time a value above 20 M results, the calculation should be rechecked as every
answer should be asked if it makes sense.
States of Matter.
Ask your self how many compounds you have seen with two states of matter
at least temporarily coexisting? If
you have ever taken a melting point, both the solid and liquid were present.
However, except for water, you probably have not observed substantial
amounts of both solid and liquid present at the same time.
This is quite phenomenal as there are millions of characterized compounds
but only for one of them, namely water, have you seen liquid and solid coexist.
Some might claim that their cooking oil sometimes has solid in the bottle
of liquid but cooking oil is a mixture and not a single substance.
If you have ever soldered a wire connection, the solder was melted but
again, solder is a mixture and the liquid quickly solidifies.
Since water has a large liquid temperature range (0 – 100oC)
over a mild range of temperatures, it is especially well suited for the
establishment of living systems.
Hotter temperatures often result in the decomposition of organic compounds (as
in cooking) and colder slows reactions down although this does not prohibit life
forming reactions. However, the
importance of the convenience of the liquid temperature range cannot be
understated.
 Boiling
Point of Water. Water is often
compared to compounds of molecular mass like such as methane (CH4),
ammonia (NH3) and hydrogen fluoride (HF).
These three are gases at room temperature.
Seldom discussed, it should be noted
that LiH and BeH2 are solids (BH3 is not included in this
discussion as it dimerizes to B2H6) with even lower
molecular mass values than water.
However, LiH is best considered to have ionic bonding compared to the covalent
bonding in methane, ammonia, water and hydrogen fluoride.
BeH2 does have covalent bonding but has a two center, three
electron bond making it rather unique and not a good model for comparison.
Boiling
Point of Water. Water is often
compared to compounds of molecular mass like such as methane (CH4),
ammonia (NH3) and hydrogen fluoride (HF).
These three are gases at room temperature.
Seldom discussed, it should be noted
that LiH and BeH2 are solids (BH3 is not included in this
discussion as it dimerizes to B2H6) with even lower
molecular mass values than water.
However, LiH is best considered to have ionic bonding compared to the covalent
bonding in methane, ammonia, water and hydrogen fluoride.
BeH2 does have covalent bonding but has a two center, three
electron bond making it rather unique and not a good model for comparison.
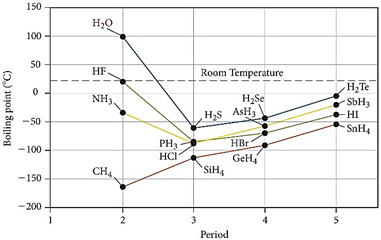
The graph on the above right
provides several features that enable meaningful conclusions about water.
Delaying temporarily a discussion of the values of the boiling points
of water, HF and NH3, it is observed that the hydrogen compounds of
the elements going down each of the groups gradually demonstrate an increase in
boiling point as is expected for increases in molecular mass and a corresponding
increase in the number of electrons and van-der-Waals forces.
Only for methane does the trend continue going to the left to lower mass as water, HF and
ammonia have higher boiling points than should be expected based on the hydrogen
compounds further down their groups.
Clearly, water, HF and ammonia must have a property that causes their
significant deviation in trends.
All three have polar covalent bonding but the effect is larger than can be
accounted for based on common polarity factors.
When hydrogen is bonded to F, O or N, a special very strong attraction
called hydrogen bonding is possible.
Very roughly hydrogen bonds are about 10% of the strength of covalent
bonds but significantly stronger than most other types of intermolecular bonds.
Hydrogen bonding plays an extremely important role in chemical
interactions including a very significant role in the determination of genetics.
Hydrogen bonds connect the two strands of the double helix of DNA and
determine the pairing of nucleobases that contain the information for
repriclation of DNA.
For water, HF and ammonia,
because of hydrogen bonding, much more energy (and a higher temperature) is
required to break the intermolecular bonds resulting in boiling points much
higher than expectations based on molecular mass and polar bonds.
If the descending line from H2Te, H2Se, H2S
is extrapolated to the left, a predicted boiling point for water results of
about -80o. Hydrogen
bonding in water is responsible for a 180o increase in the boiling
point. The boiling points of HF and
NH3 are also much higher than expected.
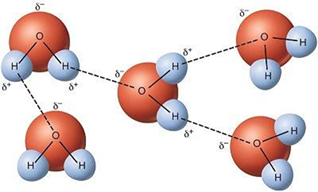 A
comparison of ammonia, water and HF reveals some inconsistencies.
The hydrogen bonding strength going across this series should increase as
the electronegativity increases the order, N, O, F.
However, this is complicated by the number of hydrogens.
For water with two hydrogens, more hydrogen bonding with a 3D structure
is possible compared to the linear HF resulting in water having a higher boiling
point than HF. For ammonia with three hydrogens, the weaker strength of the
hydrogen bonding or a less appropriate geometry apparently is more important
than an increase in the number of bonds.
A
comparison of ammonia, water and HF reveals some inconsistencies.
The hydrogen bonding strength going across this series should increase as
the electronegativity increases the order, N, O, F.
However, this is complicated by the number of hydrogens.
For water with two hydrogens, more hydrogen bonding with a 3D structure
is possible compared to the linear HF resulting in water having a higher boiling
point than HF. For ammonia with three hydrogens, the weaker strength of the
hydrogen bonding or a less appropriate geometry apparently is more important
than an increase in the number of bonds.
The importance of the liquid
temperature range of 0 – 100oC for water cannot be understated.
A glance at the liquid temperature ranges of other liquids (see:
http://murov.info/orgsolvents.htm)
reveals that most other substances with suitable liquid ranges probably would
not be too hospitable for life.
Density of Water as a Function of State of Matter.
The discussion of hydrogen bonding now leads directly to a consideration
of the densities of liquid and solid water.
The ratio of the two density values is perhaps the most unusual and
difficult to predict property of water.
While we observe ice and liquid water mixtures almost every day, few stop
to notice the unexpected and very unusual behavior.
As mentioned earlier, water is probably the only substance out of
millions that you have observed with both liquid and solid states present so
that you could notice if the solid sinks or floats in its own liquid.
Because it is the only example encountered, most accept the situation as
the norm and do not question the very discrepant event.
Why was the term discrepant used?
Any observation that differs from expectations should be questioned.
Often discrepant observations lead to useful new concepts and
explanations and should not be overlooked.
And yet, the unusual behavior of the ice water system is not noticed by
almost everyone.
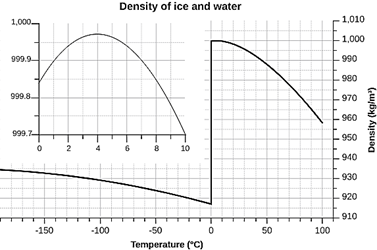

Starting from scratch, ask if you would expect the solid of a substance
to float or sink in its own liquid.
As liquids cool, it is expected and almost always true that the volume
contracts. Since density is mass
divided by volume, a decrease in volume should lead to an increase in density.
This trend should continue as the substance freezes resulting in a solid
with a higher density than the liquid and the solid should sink.
For millions of substances, this prediction is realized.
Water is one of the very few substances that behaves contrary to
predictions. In the image of 6 test
tubes, which one contains water?
Only the 3rd one has the solid floating in its liquid and must be
water.
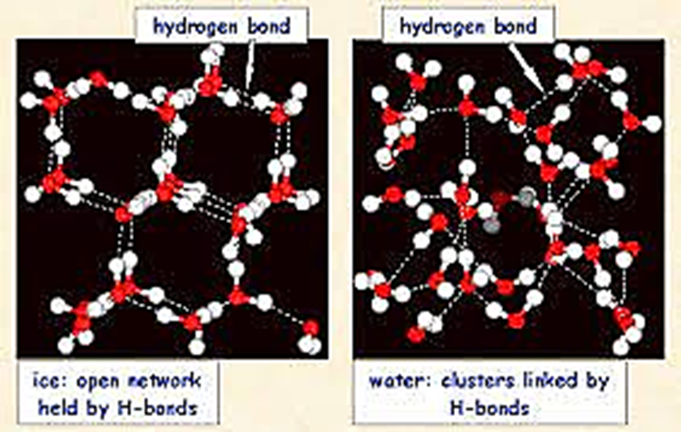
As can be observed in the images above, water molecules in ice have organized
into a 3D structure that results in the water molecules being farther apart than
they are in liquid water. It is
interesting to speculate if life would be the same if water behaved as expected.
Where would freezing take place in fresh water lakes in the winter and
would life be possible for this situation?
As discussed above, the use of good observational skills often leads to important discoveries. The quotes below from an online PowerPoint presentation http://murov.info/OpticalIllusionsandObservations.ppt emphasize the value of being prepared and careful with observation.

 In addition,
there are quotations by Sherock Holmes (authored by Arthur Conan Doyle) that are
also relevant.
In addition,
there are quotations by Sherock Holmes (authored by Arthur Conan Doyle) that are
also relevant.
There is nothing more deceptive than an obvious fact.”
Don't Just See, Observe!
The world is full of obvious things which nobody by any chance ever observes.”
There are 5 elements (arsenic, bismuth, gallium, germanium and silicon)
that exhibit the liquid-solid density reversal with only gallium having a
melting point near room temperature.
There are undoubtedly compounds in addition to water that have this
unexpected property, but it is difficult to locate data tables containing liquid
and solid densities.
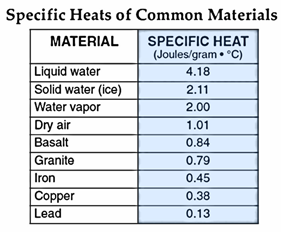
Specific Heat. Another
property of water that is commonly cited as being very unusual is the specific
heat of water. It takes 1 cal (or
4.18 joules) to heat one gram of water 1oC.
As you can observe in the table, this value is relatively high compared
to other substances.
 Like
the density reversal, the high value of the specific heat of water affords some
advantages. The same amount of
energy transferred to 100 g of iron will heat the iron over nine times as many
degrees as when transferred to 100 g of water.
In effect, this means that water is more resistant to temperature change
than most materials. Is it possible
to explain the high value for water?
Like
the density reversal, the high value of the specific heat of water affords some
advantages. The same amount of
energy transferred to 100 g of iron will heat the iron over nine times as many
degrees as when transferred to 100 g of water.
In effect, this means that water is more resistant to temperature change
than most materials. Is it possible
to explain the high value for water?
The Dulong and Petit empirical relationship for the product of atomic
mass (Mm) and specific heat, (Mm)(Cp)
= 25, indicates that the
specific heat is inversely proportional to the atomic mass or proportional to
the number of moles of particles as with colligative properties.
The Dulong and Petit relationship is supported by experimental data as
demonstrated in the table below.
This means that a measurement of specific heat for an unknown element can help
identify the element.
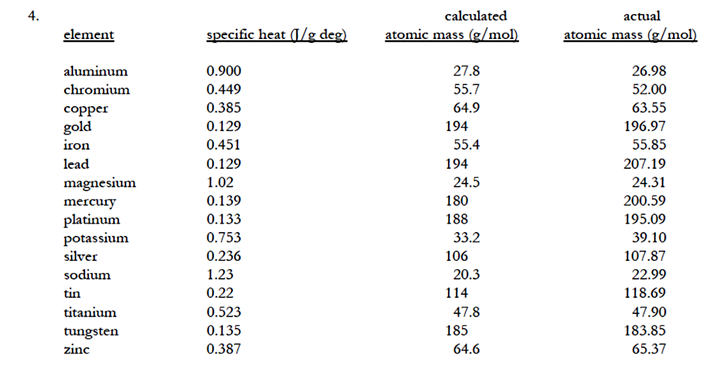
For use of the equation with molecules rather than atoms, the number 25
should be multiplied by the number of atoms per molecule.
a.
Is the value 4.184 joules/g deg. for
water consistent with this oversimplified approach (note that there is a
threshold temperature below which this approach will have significant error)?
b.
Compared to metals, what two
factors cause the specific heat of water to appear to be exceptionally high?
As water has 3 atoms the
constant becomes 3 x 25 = 75. The
molecular mass of water calculated from the Dulong and Petit relationship would
be 75/4.184 = 17.9 g/mol. This is
remarkably close to the actual value of 18.0 g/mol providing support for the
modified Dulong and Petit relationship.
The specific heat of water is larger than for other chemicals because of
the low molecular mass of water and the 3 atoms/molecule and apparently should
not be considered discrepant. While
the observation of the large value might initially give the impression of
weirdness, the value is consistent with expectations.
c. Does this concept work on
other substances such as sodium chloride, potassium chloride, calcium chloride, aluminum chloride, carbon tetrachloride or methanol (CH3OH)?
Explain your answer.
Water as a Solvent.
Water
has several more important life affecting properties such as
surface tension that appear to make water distinctive.
However, this discussion will not cover other properties but will conclude with a discussion of water as a
solvent. Many discussions of water describe
it as a universal solvent. That would be extremely weird as it implies that
water will dissolve polar and non-polar molecules as well as small and large
ones. The statement that water is a universal solvent is a huge misnomer. Since water is a small and
very polar solvent, small polar molecules tend to dissolve in water.
Solubility is very difficult to predict with the statement “like
dissolves like” about as good as you can get.
While a good solvent for many ionic compounds, there are many such as
AgCl and CaCO3 that do not dissolve substantially in water.
Water is generally not a good solvent for organic compounds that contain
five or more carbons and it is a poor solvent for most non-polar organic
compounds. Of the millions of known
compounds, most are organic and will not dissolve significantly in water.
The claim of “universal solvent” to have any meaning must be put into
context. There are probably many
solvents that dissolve more compounds than water does such as ethanol.
Conclusions.
In summary, on the surface, many of the properties of water appear to be
distinctive and perhaps unexpected.
The answer to the question “is water weird?” depends on perspective.
When properties such as a very low molecular mass are taken into account,
some of the mysteries can be explained.
Perhaps the property that would be the hardest to predict is the reversal
of the liquid to solid density ratio.
Unless you have some insight into the structures of the solid and liquid phases
that result from optimization of hydrogen bonding, this observation would
generally not be predicted.
Otherwise, the unusual properties such as specific heat are consistent with
basic principles of chemistry. If
WEIRD means different or unique, then, yes, water is weird.
But if weird means unpredictable, then water is not very weird.
However, The apparently unusual appearance of the properties gives rise
to questions and helps to make the field of chemistry intriguing and
interesting.
For web sites that discusses
other compounds with unusual properties, please visit:
http://murov.info/FascinatingChemicals.ppt
RESOURCES FOR THE
PROPERTIES OF WATER
https://byjus.com/chemistry/physical-and-chemical-properties-of-water/
https://en.wikipedia.org/wiki/Properties_of_water
https://www.worldatlas.com/articles/5-unique-properties-of-water.html
https://www.legit.ng/1394221-10-important-properties-water-about.html
https://water.mecc.edu/courses/ENV211/lesson10.htm
For many more web sites by Steven Murov ( murovs@yosemite.edu ) including several on chemistry, please visit http://murov.info .
Site first posted and hit counter started on 5/17/22