 SPOTLIGHTING
SPOTLIGHTING
 SPOTLIGHTING
SPOTLIGHTING
IMPORTANT CHEMISTRY TOPICS
These chemistry vignettes were originally written
as page fillers for Experiments and Exercises in Basic
Chemistry, S. Murov and B. Stedjee, Wiley (7 editions).
Because the lab book is no longer undergoing revision and sales have dropped to
an extremely low level, the vignettes have been posted below for educational
use. The only new one is the last one on PFAS. For many more
chemistry sites by Steven Murov, please vist:
http://murov.info.
For two sites related to the topics here, please visit:
http://murov.info/wat.htm
,
http://murov.info/FascinatingChemicals.ppt
CONTENTS
CLIMATE CHANGE, CO2, ARGON AND INERT GASES
CHEMICALS AND SOCIETY, ANTIBIOTICS
****************************************************************************************************************************************

The element titanium was discovered in 1791 by Gregor and
named in 1795 by Klaproth from the Latin, Titans, or the first sons of the
earth. This could be considered an appropriate name as titanium is the ninth
most abundant element in the earth’s crust (0.63% by weight). More impressively,
it ranks second to iron in abundance among the transition metals. Titanium is
significantly stronger than aluminum and only 1.7 times as dense. It is
comparable i n strength to iron but has only 60% of the density of iron. When
pure, titanium is a lustrous, white metal that is easily fabricated and has
excellent corrosion resistance. Unlike many other metals whose properties
deteriorate with increasing temperature, titanium retains most of its properties
up to several hundred degrees Celsius. With these wonderful properties, why do
we find its use limited to high speed aircraft, rockets, golf clubs bicycle
frames and other applications where cost is not the primary consideration?
n strength to iron but has only 60% of the density of iron. When
pure, titanium is a lustrous, white metal that is easily fabricated and has
excellent corrosion resistance. Unlike many other metals whose properties
deteriorate with increasing temperature, titanium retains most of its properties
up to several hundred degrees Celsius. With these wonderful properties, why do
we find its use limited to high speed aircraft, rockets, golf clubs bicycle
frames and other applications where cost is not the primary consideration?
Titanium occurs in nature primarily as two ores, rutile (TiO2) and ilmenite
(FeTiO3). The elemental form of titanium is produced from rutile using the
following sequence of reactions:
TiO2(s) + 2 C(s) + 2 Cl2(g) = TiCl4(g) + 2 CO(g)
TiCl4(g) + 2 Mg(s) = Ti(s) + 2 MgCl2(s)
This reaction sequence and alternatives all involve use of expensive reagents.
Thus, despite the abundance of titanium and its useful properties, titanium
remains today too expensive for most applications. However, there is hope for
less expensive titanium in the near future (see:
Titanium - Wikipedia,
How titanium is made -
material, manufacture, making, history, used, processing, parts, components,
composition (madehow.com)
Recent
Progress in Titanium Extraction and Recycling | SpringerLink
On the other hand, because of the abundance and properties of rutile, TiO2 is
used frequently. TiO2 is brilliantly white, very stable, opaque, and very
importantly, has low toxicity. These properties make it an ideal pigmentation
for paints and the annual production in the United States is near one million
tons. Unfortunately, many paints used to contain lead compounds. Some children
still get lead poisoning from eating peeled-off paint chips.
CLIMATE CHANGE, CO2, ARGON and INERT GASES
Most people, when asked what the three most abundant
gases in the atmosphere are include carbon dioxide among their answers. They
believe this because plants use CO2 and animals exhale it. Actually, the air
exhaled by humans is only about 3% CO2 and plants are able to get enough CO2 for
their needs despite the fact that CO2 ranks a distant 4th (not counting water)
in atmospheric abundance at only 0.041%.
Because the amount of carbon dioxide in the atmosphere is so low, the burning of
fossil fuels to produce primarily carbon dioxide and water has been able to
significantly increase the amount of carbon dioxide in the atmosphere (by about
47% and increasing). As carbon dioxide contributes to
the so-called "greenhouse effect," scientists are now very concerned with strong
supporting evidence that our
burning of fossil fuels will lead to a warming of the earth by a few degrees and
potentially very damaging changes in microclimates. The evidence that this is
already happening is substantial. The average temperature on earth has
risen more than 1oC, sea levels are rising, glaciers are melting and unusual and
freaky weather is occurring so frequently that it is no longer unusual or
freaky. Heat waves, wildfires, damaging storms with severe flooding are
causing many deaths, destroying our environment and causing an alarmingly
increasing extinction rate of animals. In addition, ocean water
temperature increases are destroying the coral reefs and more absorbed carbon
dioxide is acidifying the water threatening many kinds of ocean life.
Agriculture is not immune and a critical shortage of food could result.
Food and resource shortages could eventually lead to the loss of civility as
society members are forced to fight to survive. What alternative
energy sources could be substituted for fossil fuels in the event we find that
this is a real threat to the future of society?
The graph below show the excellent correlation of CO2 production, CO2 atmospheric concentration and global temperatures. Without the production curve, it could be argued that increasing temperatures are causing the increase in atmospheric CO2. However, the correlation of production and concentration provide very strong evidence that human use of fossil fuels are causing the increase in temperature and the resulting damage to our environment. Humanity faces crisis frequently but most can be remedied in months or a few years. This crisis however, will take decades to combat with untold consequences while society develops a plan to minimize the effects of 200 years of fossil fuel combustion.
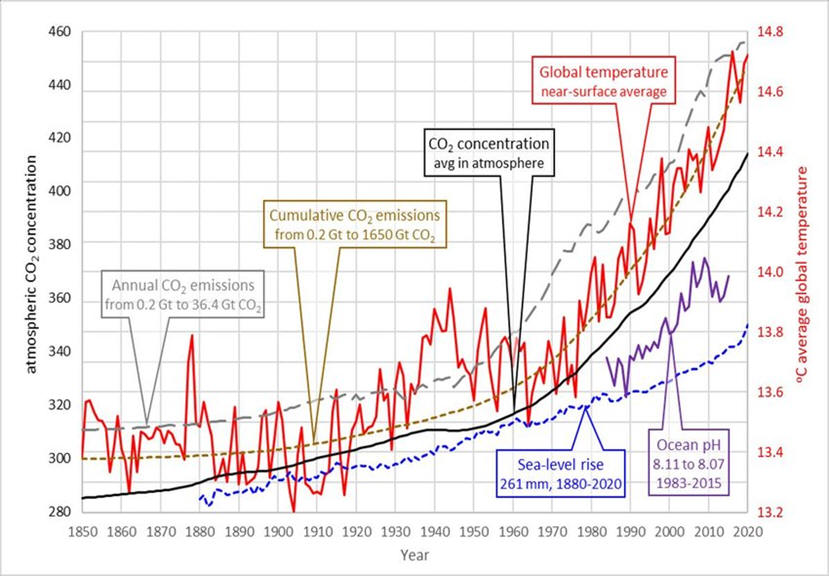
You are probably familiar with the solid state of CO2, so-called dry ice. At
-78.5oC, dry ice undergoes a phase change (called sublimation) directly into the
gas phase. To get liquid carbon dioxide, the dry ice must be under a pressure of
several atmospheres. Then warming of the dry ice results in melting instead of
sublimation.
You should also recognize carbon dioxide as the bubbles in carbonated soda and
the chemical in many fire extinguishers. Why is carbon dioxide useful in fire
extinguishers?
Argon (the r is missing or gone) is the third most
abundant gas (not counting water which varies) in the atmosphere (volume
percentages; nitrogen - 78.09%, oxygen - 20.95%, argon - 0.93%, carbon dioxide -
0.04%). The name argon is derived from the Greek, argos, which appropriately
means lazy or inactive. Argon, along with helium (Greek - sun), neon (Greek -
new), krypton (Greek - hidden), xenon (Greek - stranger) and radon (ray), make
up the group or family of elements called inert or noble gases. As Mendeleev's
first version of the periodic table was developed in 1869, only a few months
after the discovery of the first inert gas (helium) in the sun, inert gases were not
included in the first periodic table. In fact, most of the other inert gases
were not discovered until 1898 by Sir William Ramsay.
In 1962 Neil Bartlett demonstrated that inert gases are not completely inert
when he synthesized XePtF6. Shortly thereafter, chemists were able to make
several fluorides of xenon and krypton.
Because of their extremely low chemical reactivity, the inert gases have many
important practical uses. Neon is used in "neon" lighting and argon is used to
provide an inert atmosphere in electric light bulbs and for chemical reactions.
The wavelength of an orange-red line in the atomic spectrum of krypton is used
to define the meter. Helium, because of its low density, is used for balloons
and blimps. It is sometimes mixed with oxygen as a breathing mixture for divers.
Perhaps even more important, helium's very low boiling point of 4.2 K makes
liquid helium a commonly used coolant when very low temperatures are needed
(e.g., to provide a temperature where some materials become superconducting).
On a lighter side, krypton is probably most commonly recognized as the name of
Superman's home planet. The substance, "kryptonite," is said to be a green solid
that is potentially lethal to Superman but harmless to humans. In Superman III,
the computer scientist (portrayed by Richard Pryor) analyzed "kryptonite" and
found it to consist of 15.08% plutonium, 18.06% tantalum, 27.71% xenon, 24.02%
promethium, 10.62% dialium, 3.94% mercury and 0.57% unknown. Would this mixture
be harmless to humans? (for more information on fictional elements, please
visit: http://murov.info/fictionalelements.htm
Until at least 1828, organic chemistry was considered the
chemistry of compounds derived from living things. It was believed that such
chemicals had a special property called "vitalism" associated with them that
differentiated these compounds from inorganic compounds. In 1828, Wöhler
demonstrated that the organic compound, urea, could be made from inorganic
chemicals. This led eventually to the redefining of organic chemistry to the
chemistry of compounds that contain carbon. Consider then that we have one
branch of chemistry for carbon compounds and another for all of the other
elements. This is basically due to the importance of carbon in living things.
Its importance is attributable to the fact that carbon is tetravalent (forms
four bonds per carbon) and forms strong bonds to many other elements and itself
(resulting in the formation of chains and polymers).
Contrary to the beliefs of people who preceded Wöhler and people today who buy "natural" products such as natural Vitamin C
rather than the less expensive synthetic chemical, natural and synthetic
chemicals are absolutely identical.
Steel, because of strength and low cost, is one of the
primary construction materials. Steel is an alloy that consists predominantly of
iron and up to a few percent of other elements including carbon and manganese.
Stainless steel also includes significant percentages of chromium and nickel.
Unfortunately iron is susceptible to reaction with oxygen in the presence of
water to form rust (Fe2O3. nH2O). The number of waters associated with the
iron(III) oxide varies and has been represented by n in the formula. Rusting
significantly corrodes and weakens the steel. It is sometimes necessary to take
measures to prevent rusting such as galvanizing or painting.
As iron has two common oxidation states, +2 [iron(II) or ferrous] and +3
[iron(III) or ferric], it is not surprising that there is a second oxide of
iron, FeO. It is surprising however, that there is a third oxide of iron with
the formula Fe3O4. This oxide is sometimes called ferrosoferric oxide and occurs
in nature as the mineral magnetite. An oxidation number calculation results in a
value of +2 2/3 for the iron in magnetite. This probably means that two of the
three irons in Fe3O4 are +3 and the remaining one is +2.
Magnetite is sometimes called lodestone and as indicated by its name is a
ferromagnetic material. Most magnets are alloys containing over 50% iron and
varying amounts of aluminum, nickel, cobalt, and copper. A compound of the
element neodymium (Nd2Fe14B) is one of the strongest ferromagnetic materials for
magnets.
While ozone is an undesirable gas in the lower atmosphere, it has a very
important and useful role in the upper atmosphere. Ozone absorbs light from the
sun in the ultraviolet region that is capable of causing considerable damage to
living things. For humans, a decrease in ozone permits more skin cancer-causing
ultraviolet to reach us. For plants, the impact is not completely known but an
increase in ultraviolet could significantly affect the life cycle of plankton.
As plankton is at the top of the food chain, this kind of an effect would affect
all life on earth.
Like ozone, freons also have useful properties. Because they are extremely inert
and have ideal properties as refrigerants, freons have been used extensively in
all kinds of cooling equipment including air conditioners and refrigerators.
Unfortunately, the inertness causes a significant problem. Due to their great
stability, freons do not react when released in the atmosphere and after a
period of several years diffuse up in the atmosphere to the ozone layer. In the
upper atmosphere, freons absorb ultraviolet light that causes their
decomposition. The decomposition products initiate a chain reaction that leads
to the destruction of ozone.
| Freons are CFC's (chlorinated fluorocarbons) with molecules having one or more hydrogen atoms replaced by halogens (chlorine and/or fluorine). fluorotrichloromethane; CFC-11; R-11); Freon 12; dichlorodifluoromethane; CFC-12; R-12); Freon 22 ; chlorodifluoromethane; R-22; HCFC-22); Freon 113; trichlorotrifluoroethane; CFC-113; R-113). |
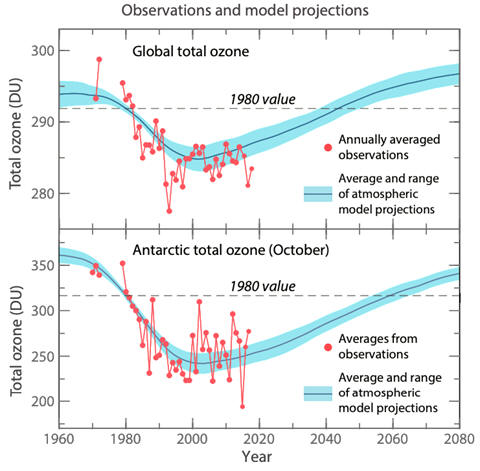 There is strong scientific evidence that the ozone concentration has decreased
on a global scale. In locations such as the Antarctic, ozone decreases annually
to the point where an ozone hole is produced. The damage already has probably
caused an increase in skin cancer, but potentially even more dangerous
will be the effect on our food chain. Most countries have signed an agreement
to phase out the use of freons in the late 20th century. As can be
observed in the graphs, the decrease in ozone has leveled as a result of the
decrease in freon use and shows some signs of slowly recovering.
There is strong scientific evidence that the ozone concentration has decreased
on a global scale. In locations such as the Antarctic, ozone decreases annually
to the point where an ozone hole is produced. The damage already has probably
caused an increase in skin cancer, but potentially even more dangerous
will be the effect on our food chain. Most countries have signed an agreement
to phase out the use of freons in the late 20th century. As can be
observed in the graphs, the decrease in ozone has leveled as a result of the
decrease in freon use and shows some signs of slowly recovering.
To fluoridate or not to fluoridate a water supply is a
question that has puzzled and even agitated many communities. There is not much
doubt that fluorides afford some protection against tooth decay. Teeth are
protected by a 2 mm layer of a mineral called hydroxyapatite, Ca5(PO4)3OH.
Hydroxyapatite slowly dissolves or demineralizes according to the following
equation:
Ca5(PO4)3OH(s) = 5 Ca2+ + 3 PO43- + OH-
The body fights this decay by running the reverse of the above reaction or
mineralization and a balance is hopefully maintained.
However, some foods, especially those with high sugar content, break down in the
mouth to produce acids such as acetic acid and lactic acid. The acids react with
the base produced in the demineralization reaction to produce water. This causes
the demineralization reaction to shift to the right, resulting in a loss of
protective enamel. Without the enamel, tooth decay can begin.
With fluoride present, the following reaction can take place:
5 Ca2+ + 3 PO43- + F- = Ca5(PO4)3F(s)
As the product fluorapatite contains fluoride rather than hydroxide, it is more
resistant to acid and helps to protect the teeth.
As with all other chemicals, too much is not good either. While fluoride is safe
at the concentrations needed to provide protection, higher levels cause mottled
teeth and are toxic. Fluoride is present as SnF2 or NaF in many toothpastes.
Dentists give fluoride treatments for teeth and some municipalities fluoridate
water supplies. The total exposure to fluoride from these sources should be kept
at a useful but safe level.
Among the top industrial chemicals produced in the
United States each year are four that are important because of their acid or
base properties. The acids are sulfuric acid (H2SO4) and phosphoric acid (H3PO4)
and the bases are sodium hydroxide (NaOH) and ammonia (NH3). Part of the need
for acids and bases results from the observation that hydrogen ions or hydroxide
ions function as general catalysts for many reactions. Other reactions consume
hydrogen or hydroxide ions. In addition, the acids and bases are important in
many other processes including metallurgy, petroleum refining and the synthesis
of fertilizers, plastics, detergents, glass, drugs, dyes, paint, paper and
explosives.
Other chemicals high ranking in production are nitrogen, oxygen, chlorine and
two organics, ethylene and propylene. Among many applications, nitrogen is used
for the synthesis of ammonia which in turn is used for the synthesis of nitric
acid. Oxygen is used predominantly in the steel industry but is also used in
chemical manufacture, sewage treatment and rocket fuel. Chlorine is used
principally in water treatment, bleaching and chemical manufacture. Ethylene
(C2H4) and propylene (C3H6) are used in polymer synthesis and in the preparation
of many other organic chemicals.
Of the remaining chemicals in the top 50, over half are organic. Many of the
organics such as acetone (solvent) are themselves of use. Others are used for
the synthesis of additional organic chemicals such as polymers and medicines.
One organic compound that zoomed up in the top 50 during the early 1990s was
methyl tert-butyl ether [MTBE - (CH3)3COCH3]. Because of its high octane rating
and oxygen content, MTBE was used as a replacement for the use of lead compounds
in gasoline. Unfortunately, MTBE has a significant solubility in water. Because
many gasoline storage tanks leak, water supplies were becoming contaminated with
MTBE. MTBE has been phased out and primarily replaced with ethanol.
Included among the remaining inorganic chemicals in the top 50 are ammonium
nitrate (fertilizers, explosives), carbon dioxide (refrigerant, beverage
carbonation), hydrochloric acid (chemical manufacturing) and titanium dioxide (paint pigment).
Isotopes are atoms of the same element with different
numbers of neutrons. Nuclear stability depends on the proton to neutron ratio
and determines if an atom is radioactive and if so, the type of radioactive
decay. Chemical reactivity does not depend significantly on the proton to
neutron ratio although there are small but measur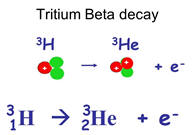 able effects on reaction rates.
One notable exception involves the isotopes of hydrogen. These isotopes and
their differences are important enough for them to earn their own names. 99.98%
of the naturally occurring hydrogen atoms consist simply
of 1 proton (and one electron) . The remaining 0.02% of hydrogen atoms also have a stable nucleus
consisting of 1 proton and 1 neutron. This isotope is called deuterium. A third isotope of
hydrogen, tritium (H), has 1 proton and 2 neutrons and undergoes beta decay with
a half life of 12.5 years.
able effects on reaction rates.
One notable exception involves the isotopes of hydrogen. These isotopes and
their differences are important enough for them to earn their own names. 99.98%
of the naturally occurring hydrogen atoms consist simply
of 1 proton (and one electron) . The remaining 0.02% of hydrogen atoms also have a stable nucleus
consisting of 1 proton and 1 neutron. This isotope is called deuterium. A third isotope of
hydrogen, tritium (H), has 1 proton and 2 neutrons and undergoes beta decay with
a half life of 12.5 years.
If a chemical reaction involves the breaking of a bond between an atom such as
carbon or oxygen and hydrogen, the reaction proceeds about seven times slower
with 2H (2D) than with 1H. This means that drinking 100% D2O instead of H2O would
considerably disrupt the balance of reactions in your body and too much heavy
water could cause
serious health effects. The difference in chemical behavior of the hydrogen isotopes should not
be surprising considering the fact that the addition of a neutron to H doubles
its mass. For other elements, the mass differences are usually small percentages
of the total mass.
| H2O | D2O | |
| boiling point (oC) | 100.00 | 101.42 |
| melting point (oC) | 0.00 | 3.81 |
| density (g/mL) (25oC) | 0.9970 | 1.1044 |
Ethanol or ethyl alcohol (C2H6O) is recognized by most as
the intoxicating compound in alcoholic drinks. In hard liquor such as vodka,
ethanol is usually about 40% by volume (80 proof), wine consists of about 12%
alcohol and beer is about 6% alcohol. Ethanol is produced by the anaerobic
fermentation (in the presence of oxygen, acetic acid is produced instead of
ethanol) of glucose by yeast:
C6H12O6(aq)
g 2 C2H6O(aq) + 2 CO2(g)
glucose
ethanol
Fermentation yields a maximum of 15% ethanol because the ethanol denatures the
yeast enzyme. The solution is distilled to achieve higher concentrations of
ethanol. Surprisingly, even distillation yields a maximum of 95% ethanol. This
is due to the fact that ethanol and water form a constant boiling mixture called
an azeotrope that consists of 95% ethanol and 5% water. To obtain 100% ethanol
from this mixture requires the use of other techniques.
Alcohols containing three or fewer carbons are miscible with water and can even
be thought of as derivatives of water. If one of the hydrogens of water (HOH) is
replaced with an alkyl group (CnH2n+1) such as ethyl, an alcohol results. The
simplest alcohol, methanol (CH4O), is also called wood alcohol. In the body,
methanol is oxidized to the very toxic chemicals, formaldehyde and formic acid.
As little as 30 mL (1 oz) of methanol can be lethal. Smaller amounts can lead to
blindness. Deaths have resulted from the substitution of methanol for ethanol.
Ethanol is metabolized by an enzyme in our bodies called alcohol dehydrogenase
to acetaldehyde. It is believed that acetaldehyde is at least partially
responsible for hangovers.
Ethanol has many other uses in addition to alcoholic beverages. For example, it
is used in organic synthesis, as a solvent and as a gasoline additive.
The elements oxygen (65%), carbon (18%), hydrogen (10%),
nitrogen (3%), calcium (1.5%), phosphorus (1.2%), potassium (0.2%), sulfur
(0.2%), chlorine (0.2%), sodium (0.1%) and magnesium (0.05%) compose more than
99% of the human body. In addition, there are trace but essential amounts
(<0.05%) of iron, cobalt, copper, zinc, iodine, selenium and fluorine. Aluminum,
bromine, chromium, manganese, molybdenum and silicon are also found in the human
body.
While small amounts of the trace elements are needed, the range of
concentrations tolerated by the body is often narrow. This means that
consumption of excessive supplements of the trace elements can cause more harm
than good.
Cobalt ion, is a
case in point. Vitamin B12 is a coordination complex of cobalt and plays a role
in the synthesis of nucleoproteins, proteins and red blood cells and the
functioning of the nervous system. Deficiency of vitamin B12 leads to pernicious
anemia. Only minute amounts are needed but its only source is from animal
products including milk. Absolute vegetarians should supplement their diets with
vitamin B12. Cobalt ion has an LD50 on the order of 0.2
g/kg, thus about 12 g of cobalt ion can be fatal and excesses of vitamin B12 should be avoided.
In one of the most elegant and difficult accomplishments of organic chemistry,
the late Dr. R. W. Woodward and a team of chemists synthesized vitamin B12 in
1973. For his very significant contributions to the field of synthetic organic
chemistry, Dr. Woodward was awarded a Nobel prize in chemistry.
HEAVY METAL POISONING
On November 23, 2006, Alexander Litvinenko, an ex-Russian spy, died as a result
of polonium-210 poisoning. Because it is extremely difficult to obtain the very
dangerous and toxic polonium-210 (about a million times more toxic than hydrogen
cyanide or an LD50 of 5x10-8 g), it is certain that Litvinenko was intentionally
killed. During the last millennium, there have been many other deaths that have
been caused by the toxic compounds of certain elements. The most common toxic
elements are the heavy metals mercury, arsenic, antimony, lead and thallium.
While some of the deaths have been the result of intentional poisoning, other
deaths have been the result of inappropriate use of chemicals. In many cases,
the chemical was being used for medicinal purposes with the user unaware of the
potentially toxic side effects of the chemical. It is difficult to establish the
cause of death for people who died hundreds of years ago but in many cases
recorded symptoms were consistent with heavy metal poisoning. In addition, hair
samples have been preserved from some famous people. Analysis using modern
methods has revealed extraordinarily high amounts of heavy metals in many of
these people. A partial list of people who suffered from the chronic effects of
poisoning and may have died as a result is included below:
Isaac Newton - Demonstrated occasional madness associated with mercury
poisoning. Hair samples showed high mercury, lead, arsenic and antimony levels
perhaps as a result of his alchemical studies.
King Charles II - Probably died from breathing mercury vapor from a chemistry
laboratory in his house.
Napoleon - Hair samples showed high mercury but he probably died from arsenic.
Sir Thomas Overbury - the poet was murdered with mercury compounds.
Charles Darwin - Probably suffered from chronic arsenic poisoning as a result of
its use for medicinal purposes
Mozart - Antimony compounds are suspected as cause of his death.
Handel and Beethoven - Both probably suffered from chronic lead poisoning.
King George III - Suffered from lead and arsenic poisoning.
Pope Clement II - Might have been poisoned with lead compounds.
For more information on the deaths referred to above as well as great reading on
other poisonings, read: Emsley, J., The Elements of Murder, Oxford, 2005.
CHEMICALS AND SOCIETY, ANTIBIOTICS
The next time you have the opportunity, ask some
elementary school children if they use any synthetic (made by humans) chemicals.
You will probably be surprised to find out that many cannot think of any. When
they finally do, it is usually chemicals like make-up or hair spray. If you
probe further, the children begin to realize that they are surrounded by
synthetic chemicals. Eventually the students usually name cleaning agents,
plastics, explosives, clothing materials, food additives, pesticides, computer
chips, gasoline and medicines. In fact, they then realize that it is hard to
name "natural" chemicals that they use.
Despite the fact that the advantages of synthetic chemicals far outweigh the
disadvantages, many people focus only on the problems caused by chemicals and
never notice how dependent they are on the beneficial aspects of chemicals.
While it is true that pesticides such as DDT have caused considerable damage to
our ecology, people don't realize that DDT saved millions of lives. We should,
however, make sure a chemical is safe before using it rather than waiting to
determine after using it that it has detrimental results.
Before World War II, it was common for bacterial infections to be life
threatening. Although Fleming discovered that penicillin kills bacteria in 928,
it took 15 years before scientists finally developed ways of effectively
administering penicillin. Since then we have lived in an era with bacterial
diseases basically under control. Recently, however, it has been found that some
bacteria have mutated to the point that very few antibiotics are effective
against them. We need to very quickly develop new antibiotics to combat this
threat or we may enter a new era where bacteria commonly kill once again.
Apparently, the research into the development of new antibiotics is limited as
pharmaceutical companies do not think they will earn a significant profit from
their development.
Bleach (which is commonly
sodium hypochlorite) is just one of the many
chemicals found in most households. Many of these chemicals, including bleach,
are potentially very dangerous as they are corrosive and very toxic.
Many household cleaning agents contain ammonia. One should be sure that bleach
and ammonia products are never mixed as they react according to equations
including the following:
NH3(aq) + NaOCl(aq)
g NH2Cl(aq) + NaOH(aq)
2 NH3(aq) + NaOCl(aq)
g N2H4(aq) + NaCl(aq) + H2O(l)
Both the hydrazine and chloroamine produced are very toxic.
Another potentially hazardous situation can occur with the chemicals used to
prevent bacterial growth in pools and to adjust the pH. One method of
chlorinating pools is to use a sodium hypochlorite solution that is usually
supplied by pool stores at twice the concentration of household bleach. If the
pH of the pool is too high, muriatic acid (commercial name for hydrochloric
acid) is usually added to the pool. If NaOCl solution and HCl are poured into
the same location of the pool in a short amount of time, toxic chlorine gas will
be produced.
NaOCl(s) + 2 HCl(aq)
g
Cl2(g) + NaCl(aq) + H2O(s)
The two solutions should be added to different ends of the pool, preferably at
different times.
Looking around today at the human made objects all around
us, we notice that a significant number are composed of various kinds of
polymers. From our polyester clothes to styrofoam coffee cups, we are surrounded
by a world of polymeric compounds. Some people argue that styrofoam and other
polystyrene products are damaging the environment because they are not
biodegradable. The options available are not necessarily better. Production of
paper uses trees, considerable energy and paper bleaching can result in the
production of toxic chemicals. Also it has been found that paper does not
biodegrade in the kinds of landfills we currently use. Reusables such as plastic
or porcelain dishes offer many advantages over their styrofoam and paper
counterparts but even reusables cause environmental problems. It takes water and
potentially harmful detergents to clean the dishes. The message is that issues
like these are much more complicated than it might appear and the best solution
may vary from one situation to the next. Most important, consider all the
issues.
Another common polymer, polyvinyl chloride (PVC) is used for many applications
including water pipes and seat covers. The compound used to make PVC, vinyl
chloride (C2H3Cl), is highly carcinogenic. Before this was recognized many
people involved in the industrial preparation of PVC unfortunately acquired
liver cancer.
Despite occasional very unfortunate mistakes like the one with vinyl chloride,
the chemical industry is one of the safest of all industries for its workers in
terms of days lost from the job.
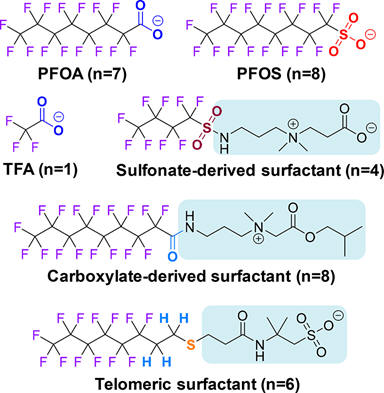
PFAS (Per- and polyfluoroalkyl substances) are human-made chemicals that don’t break down easily. That’s because they are used to make products that resist grease, oil, stains, heat, and water. PFAS chemicals can be found in numerous substances like cleaning products, waxes, paints, food packaging, fire-fighting foams, and more.
Humans and
animals can be exposed to PFAS chemicals in the air, in the soil, and in water.
This can result in
PFAS in drinking water, the environment, and even building up in fish
and other wildlife. Scientists are
still unable to figure out how long it takes for these chemicals to disappear.
That means the chance of being exposed to them is very high. In fact, a study by
the National Health and Nutrition Examination Survey (NHANES)
found PFAS in the blood of 97% of Americans.
https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm
While it’s
still unclear what the long-term effects of PFAS exposure are for certain,
research suggests high levels of PFAS
may lead to many health issues. For more information, please visit:
 https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm
https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm
Aqua Clara - What Are PFAS Chemicals?
What are PFAS chemicals, and what are they doing to our health? | CNN
White paper challenge. Each of the
following is a controversial issue currently confronting society. Choose one or
more of these topics or with the instructor’s approval, a topic of your own
design. Give the arguments pro and con on each side of the issue, state your
preference and suggest solutions to existing problems.
1. Fossil fuel issues. Focus on one aspect of the issue such as:
a. is there a correlation between the increasing atmospheric carbon dioxide
concentration and the amount of carbon dioxide introduced into the atmosphere by
combustion
b. does increasing atmospheric carbon dioxide increase the acidity of the ocean
and if so, what are the expected consequences
c. could switching from a coal and oil economy to natural gas alleviate any
problems
d. critically evaluate the editorial comments or any of the links in the site,
http://murov.info/climatechange.htm
2. Consider the fresh water supplies and needs of the world and discuss concerns
and solutions to potential problems.
3. You have probably heard that people with high blood pressure should avoid
foods that are high in sodium ion. It is possible that at least a part of the
cause of blood pressure increase is due to chloride. Explain why it is difficult
to perform a controlled study to distinguish between the blood pressure effects
of sodium and chloride and perform an Internet search to try to ascertain the
best and most recent available evidence on this issue. Is substitution of KCl
for NaCl a wise therapy? Examples of Internet sites that might be useful are:
(Links to these sites are available at: http://murov.info/exptsgenchem.htm)
http://www.nejm.org/doi/full/10.1056/NEJM199003013220901
http://www.nejm.org/doi/full/10.1056/NEJM198710223171702
http://www.ncbi.nlm.nih.gov/pubmed/15723964
4. Should metropolitan water
supplies be fluoridated?
5. Should more nuclear fission power plants be constructed?
6. Should we invest heavily in nuclear fusion research?
7. Should the NASA budget be increased or decreased?
8. Should people take vitamin C on a daily basis? Evaluate the
contributions of Linus Pauling including his advocacy for Vitamin C.
9. Should a college cafeteria use paper plates, Styrofoam plates or reusable
dishware?
10. Should nitrites be added to bacon, hot dogs, bologna, etc.?
11. Should dentists use silver amalgams to fill cavities?
12. Should we invest heavily in solar voltaic cell research?
13. Should insecticides and herbicides be used in agriculture?
14. What is the function of bisphenol A in plastics and should bisphenol A use
be allowed?
15. How serious a hazard is radon in homes?
16. Does rogaine really cause hair to grow and are there side effects?
17. What are the latest research results about the origin of the Shroud of
Turin?
18. Do sun screens help to prevent skin cancer and are there any problems with
sun screens?
19. Should we be alarmed by widespread exposure to phthalates and perchlorates?
20. Are there any problems or dangers associated with the use of the aspartame?
21. Does the consumption of caffeine positively or negatively affect your
health?
22. What are high temperature superconductors and what could they be used for?
23. What are supercritical fluids and what could they be used for?
Hit counter started 5/27/22
unique hit counter started 5/27/22