Oxidation Numbers, Electronegativity, Formal Charges, Molecular Polarities, Climate Change, pH, the Checking of Calculation Results and the Correcting of Errors.
Steve Murov, Professor Emeritus of
Chemistry, murovs@yosemite.edu
This is the second of two web sites intended to provide insight into chemical concepts. The first site entitled ATOMIC MASS and ISOTOPES: INTERPRETING THE NUMBERS [1] focused on atomic mass and the information that can be gleaned from its value. This utilitarian knowledge is not commonly discussed in texts but provides useful insight into the isotopes of each element and is worthy of students’ attention. This paper continues on with the theme that students can often gain valuable insight into chemistry concepts by thinking beyond the box or reading between the lines. Applications to oxidation numbers, formal charges, molecular polarities, climate change, pH, the checking of calculation results and the correcting of errors are presented. John Packer, et. al. [2] and David DeWit [3] have previously presented examples on some of these topics.
For pertinent exercises,
please visit: http://exercises.murov.info/ex4-2.htm
http://exercises.murov.info/ex4-3.htm
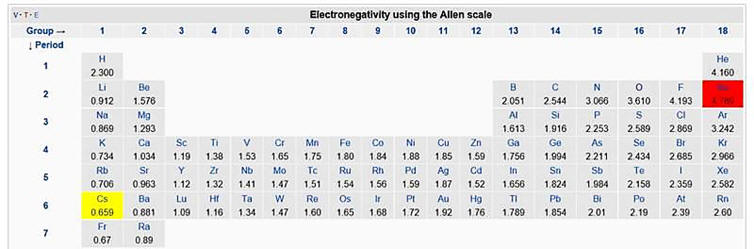
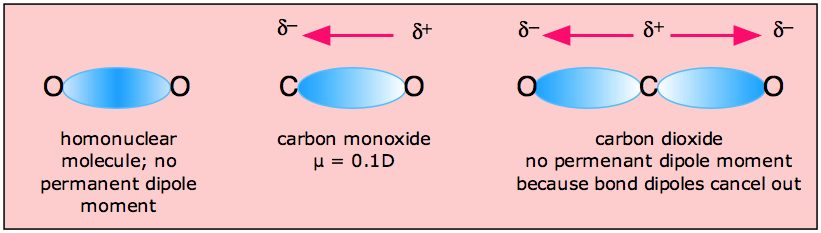
Electronegativity. The determination of bond character, that is, whether a bond is ionic or covalent, is not straightforward. Probably the most common method relies on the use of a comparison of the electronegativity values of the bonding partners. The use of electronegativity to determine the percent ionic character results in substantial uncertainty for several reasons. First, there are several different electronegativity scales with significant differences in values. As a result, the percent ionic character determined from the scales can vary substantially. The primary complication is that few bonds are either 100% ionic or 100% covalent. Several formulas have been developed that estimate the % of ionic character from the electronegativity difference. The formulas and the resulting % ionic character for several DEN values are included below.
| % ionic equation | DEN = | 0.5 | 1 | 1.7 | 2 | 3 |
| [1 - e-(DEN/2)2] x 100 | % ionic | 6 | 22.1 | 51 | 63 | 90 |
| 16(DEN) + 3.5(DEN)2 | 9 | 19.5 | 37 | 46 | 80 | |
| 18(DEN)1.4 | 7 | 18 | 38 | 47 | 84 | |
| 29.4(DEN) | 15 | 29.4 | 50 | 59 | 88 | |
| (ENhigher - ENlower)/ENhigher |
The last equation depends not only on DEN
but also on values of EN so values cannot be caluculated with only
DEN values.
Use of the graph below provides a visual method for determining the % ionic character but demonstrates that there is substantial uncertainty about the value. Many sources claim that an electronegativity difference >1.7 can be interpreted to mean that the bond is ionic. Between 0.5 to 1.7 the bond is supposedly polar covalent and <0.5 the bond is non-polar covalent. Note, however, that CsI, LiCl and HF have similar electronegativity values but the reported % ionic character varies from about 45 to 74%.
The formulas above were developed for use with the Pauling electronegativity scale. However, three other scales have been found to correlate better with bond properties. [4] For the purposes of this web site, when EN values are needed, the Allen scale will be used. Problems still occur as carbon-sulfur and carbon-iodine bonds behave as if they are polar covalent with a partial positive charge on the carbon but these experimental observations are contradictory to DEN values. Unlike the Pauling scale, the Allen scale does appropriately have the electronegativity of nitrogen higher than for chlorine. This is consistent with the observation that nitrogen participates in hydrogen bonding but chlorine does not. Electronegativity is not the only parameter that needs to be considered when determining the % ionic character. Size of the ions and other variables are also important and not necessarily taken into account by the EN value.
Generally, it is not necessary to consult an electronegativity table and it is sufficient to conclude that ionic bonding predominates for metals to non-metals. Bonding between identical non-metals and carbon to hydrogen bonds are non-polar covalent. Polar covalent bonding predominates for non-identical non-metals to non-metals.
For problems on bonding types, please see: http://exercises.murov.info/ex6-3.htm .
Oxidation Number Calculations. Determination of oxidation numbers in a compound requires an assumption of the value of the oxidation number of one of the partners of the bond. A set of rules determines the priority for the assumptions. For a compound like NaCl, the sodium has the higher priority. Since IA elements in compounds have a (except for H) +1 oxidation number, the chlorine by calculation has to have an oxidation number of -1. As rule 2 states, the sum of the oxidation numbers in a neutral compound must be zero, 1 + x = 0 and x = -1. A later rule is that VIIA elements in binary compounds have an oxidation number of -1. For this case, the calculation is consistent with both rules. For a compound like H2O2, the prioritization of the rules becomes necessary but also is consistent with logic. When hydrogen is bonded to an element more electronegative than itself, it behaves like the other IA elements and has a +1 oxidation number. This results in a -1 oxidation number for oxygen [2(+1) + 2x = 0]. If the prioritization had been ignored and it was assumed that oxygen is -2, then hydrogen would come out +2. This is an impossible conclusion.
The rules should be used in
this order - the higher the rule, the higher its priority.
1. The oxidation number for
an element in its elemental form is zero.(e.g. I2, carbon in diamond
and graphite).
2. The sum of the oxidation
states of all the atoms or ions in a neutral compound is zero.
3. The sum of the oxidation
states of all the atoms in an ion is equal to the charge on the ion.
4.
Fluorine has an oxidation number of -1 in compounds (other than F2).
5. The O.N. of group
IA
elements is +1.
6. The O.N. of group IIA
elements is +2
7. The O.N. of oxygen is -2,
except peroxides where it is -1.
8. The O.N. of halogens is
usually -1.
9. The O.N. of hydrogen is
+1 when bonded to non-metals and -1 when bonded to metals.
10. The
oxidation numbers of polyatomic ions must be learned (e.g. nitrate in NO3-)
In summary, the least electronegative element is assigned a positive oxidation state. The more electronegative element in a substance is assigned a negative oxidation state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases faster going down than going to the left. Fluorine is the most electronegative element with an oxidation state -1 except in F2.
Examples:
AlCl3 x + 3(-1) = 0 x (O.N. for Al) = 3
Ti(NO3)4 It needs to be recognized that nitrate has a charge of -1. For the titanium x + 4(-1) =0 with x or the oxidation number of titanium = 4. Thus the name would be titanium(IV) nitrate. For the nitrogen, see the next example.
NO3- Application of the rules results in O = -2. For the nitrogen, x + 3(-2) = -1 and x = 5.
For more examples, see: http://exercises.murov.info/ex13-1.htm
The periodic table below give very approximate values of the oxidation numbers for the elements.
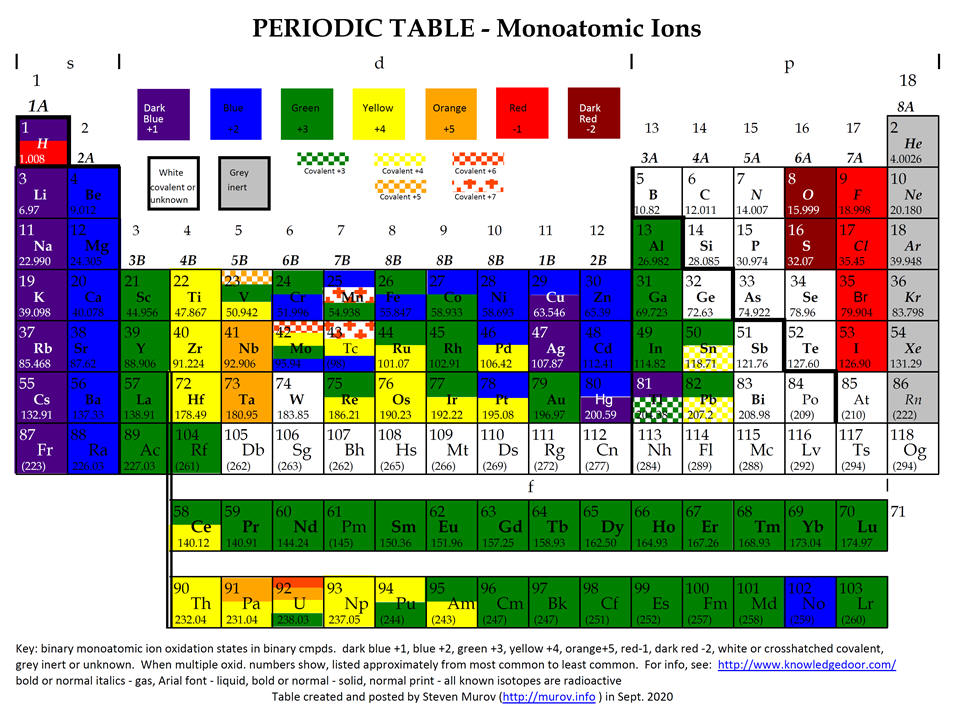
Information from oxidation numbers.
Q1.
C
formal charge = valence electrons
- bonds - nonbonded electrons
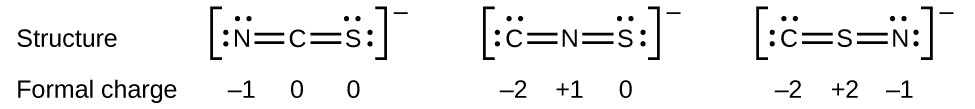
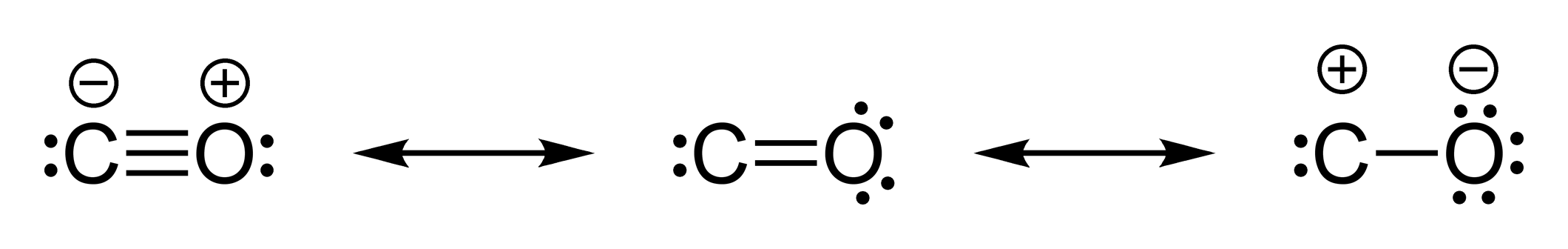
Q2. For laughing gas (N2O), use Lewis structures and a consideration of formal charges to predict if the sequence of bonding should be NNO or NON. One of the guidelines for Lewis structures is that symmetrical structures are usually favored over asymmetrical structures. Is this one of the exceptions?
Q3. In hypochlorous acid, would the H be expected to be bonded to the O or the Cl?
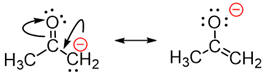
For the resonance structures of the enolate ion, the resonance structure with
the negative formal charge on the more electronegative oxygen would be expected
to be more important than the one with the charge on the carbon.


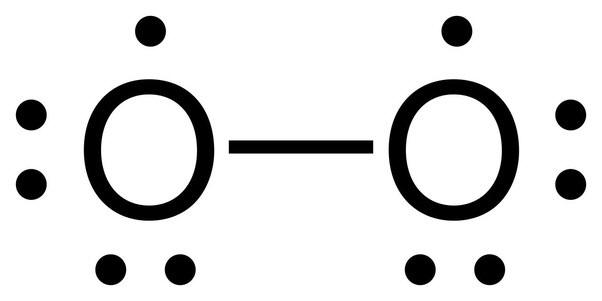
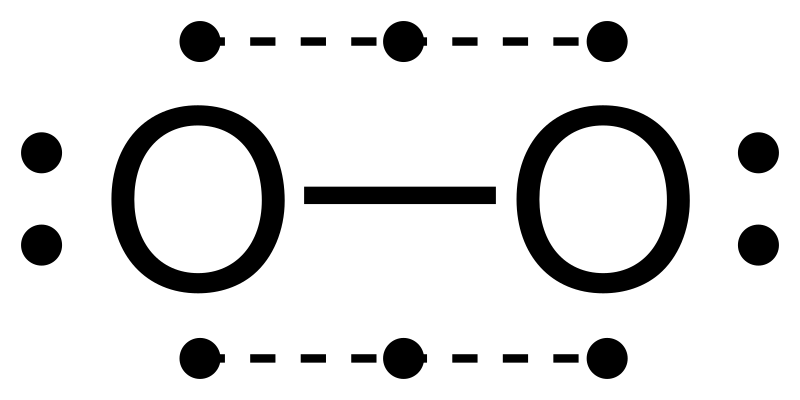
![]() This
incorrect model is one of the few failings of the Lewis structure method as liquid oxygen
when poured through a magnetic field bends towards one of the magnetic poles.
This means that O2 is paramagnetic and that it has at least one unpaired electron.
A decent and correct Lewis structure of oxygen cannot really be drawn. A molecular
orbital model
does correctly depict oxygen as a diradical with two unpaired electrons.
The structure on the left below does depict a diradical but also shows only a
single bond. Diatomic oxygen behaves as though it has a double bond. The
structure on the right goes beyond normal Lewis structure guidelines and is difficult
to interpret.
This
incorrect model is one of the few failings of the Lewis structure method as liquid oxygen
when poured through a magnetic field bends towards one of the magnetic poles.
This means that O2 is paramagnetic and that it has at least one unpaired electron.
A decent and correct Lewis structure of oxygen cannot really be drawn. A molecular
orbital model
does correctly depict oxygen as a diradical with two unpaired electrons.
The structure on the left below does depict a diradical but also shows only a
single bond. Diatomic oxygen behaves as though it has a double bond. The
structure on the right goes beyond normal Lewis structure guidelines and is difficult
to interpret.
The very hazardous pollutant, nitrogen dioxide, is a
reactive free radical. D
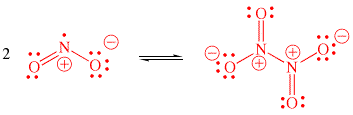


Q4. Now many unpaired electrons does each of the following have: NO, SO2, Fe, Nd, N2 ?
 |
 |
 |
 |
| solvent | formula | b.p. (oC) |
m.p. (oC) |
density (g/mL) |
relative polarity |
solubility in water (g/100g) |
dipole moment (D) |
dielectric constant |
||
| 1 | cyclohexane | C6H12 | 80.7 | 6.6 | 0.779 | 0.006 | 0.005 | 0 | 2 | |
| 2 | hexane | C6H14 | 69 | -95 | 0.655 | 0.009 | 0.0014 | 0 | 1.9 | |
| 3 | toluene | C7H8 | 110.6 | -93 | 0.867 | 0.099 | 0.05 | 0.36 | 2.4 | |
| 4 | ether | C4H10O | 34.6 | -116.3 | 0.713 | 0.117 | 7.5 | 1.25 | 4.3 | |
| 5 | ethyl acetate | C4H8O2 | 77 | -83.6 | 0.894 | 0.228 | 8.7 | 1.78 | 6.0 | |
| 6 | acetone | C3H6O | 56.2 | -94.3 | 0.786 | 0.355 | M | 2.85 | 21 | |
| 7 | 1-octanol | C8H18O | 194.4 | -15 | 0.827 | 0.537 | 0.096 | 1.68 | 10.3 | |
| 8 | 1-hetanol | C7H16O | 176.4 | -35 | 0.819 | 0.549 | 0.17 | 1.6 | 12 | |
| 9 | 1-hexanol | C6H14O | 158 | -46.7 | 0.814 | 0.559 | 0.59 | 1.60 | 12.5 | |
| 10 | 1-pentanol | C5H12O | 138.0 | -78.2 | 0.814 | 0.568 | 2.2 | 1.7 | 14 | |
| 11 | 1-butanol | C4H10O | 117.6 | -89.5 | 0.81 | 0.586 | 8.5 | 1.7 | 17.5 | |
| 12 | 1-propanol | C3H8O | 97 | -126 | 0.803 | 0.617 | M | 1.68 | 22 | |
| 13 | acetic acid | C2H4O2 | 118 | 16.6 | 1.049 | 0.648 | M | 1.68 | 6.2 | |
| 14 | ethanol | C2H6O | 78.5 | -114.1 | 0.789 | 0.654 | M | 1.7 | 24 | |
| 15 | methanol | CH4O | 64.6 | -98 | 0.791 | 0.762 | M | 1.6 | 33 | |
| 16 | water, heavy | D2O | 101.3 | 4 | 1.107 | 0.991 | M | 1.84 | 78.3 | |
| 17 | water | H2O | 100.00 | 0.00 | 0.998 | 1.000 | M | 1.85 | 78.3 |
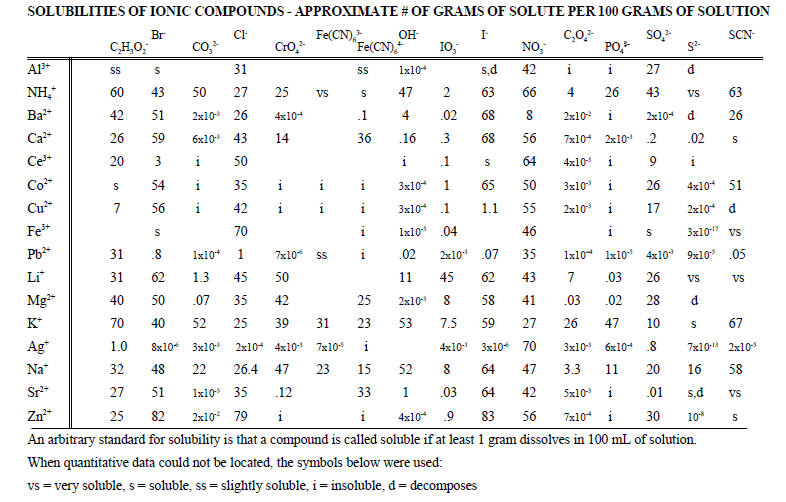
M = miscible
For more complete tables of solvent properties, please see:
http://murov.info/orgsolvents.htm,
http://murov.info/orgsolvsort.htm
As noted, the solvent table has been arranged according to increasing
relative polarity using a solvent spectral shift measurement. Solvent
polarity much like electronegativity is not a well defined property. In
addition to the relative polarity values used above, dipole moments and
dielectric constants are also used to rank polarities. The table shows
that there is a correlation of the solvent shift polarities with dipole moments and
dielectric constants with some notable exceptions.
The best
predictor of solubility is probably "like dissolves like." Because
many variables affect solubility, the best way by far to determine
solubility is to refer to the literature or experimentally determine it.
There are some trends that are predictable. For the series 1-octanol,
1-heptanol, 1-hexanol, 1-pentanol, 1-butanol, 1-propanol, ethanol and
methanol, the table shows a consistent trend towards increasing solubility
in water as the number of carbons decreases. One way to view these molecules is
to consider them to be composed of two parts, a non-polar carbon chain and a
very polar OH group. When the carbon chain is long, the non-polar
properties dominate and the compound has low solubilty in water. As
the carbon chain shortens, the polar hydroxy group becomes the dominating
part of the molecule. The three alcohols with three carbons or less
are all miscible with water.
Q5.
Should the first compound listed be more soluble
in the first solvent or the second?
a. NaCl in water or toluene
b. KOH in water or toluene
c. HCl in water or
toluene
d. wax in water
or toluene
Q6.
Using solubility differences only, suggest a method
for distinguishing between:
a. PbCl2 and BaCl2
b. BaSO4 and ZnSO4
c. acetone and
hexane
Q7.
a.
The molecular mass values for
hexane and 1-pentanol are similar. Suggest a reason that the b.p. of
1-pentanol is higher than the b.p. of hexane.
b. The boiling points
of methane, water and hydrogen iodide are -162oC, 100oC, and -35oC
respectively. Suggest an explanation for the values.
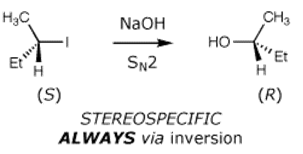
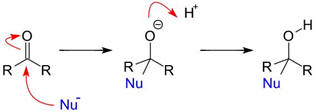
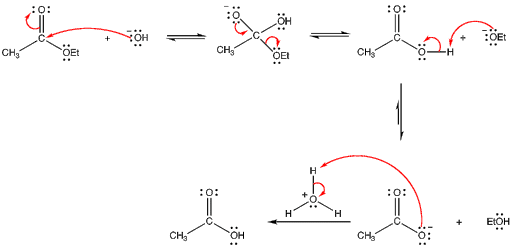
In addition to the use of molecular polarity for the
prediction of solubility,
polarity is a very useful property for the determination of the course of organic reactions. Many
reactions are initiated by the attack of a nucleophile or and electrophile.
Where should we expect a nucleophile to attack but on a site that has a
partial positive charge. Common examples are carbons bonded to a
leaving group such as a halide or protonated alcohol or to an sp2 hybridized carbon
that has a double bond to an oxygen such as a carbonyl or carboxyl group.
Electrophiles or species with positive or partially positive charges should
be expected to attack regions of high electron density such as an aromatic
ring. Examples of attack by nucleophiles on carbons with partial
positive charges are below.
Reactions initiated by nucleophiles:
 |
 |
 |
 |
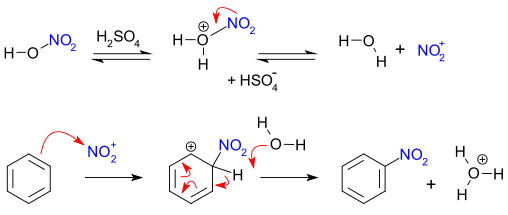 |
Climate change and pH.
Climate change is one of the most daunting challenges currently confronting all
life on earth. The first step in the effort to overcome the challenge is
to significantly increase climate change science literacy.
Thinking about the magnitudes of concentrations
can provide valuable
insight.
Most people including
those with strong opinions on both sides of the global climate debate cannot
name the three most abundant gases in
dry air.
Q8. Name the 3 most abundant gases in dry air.
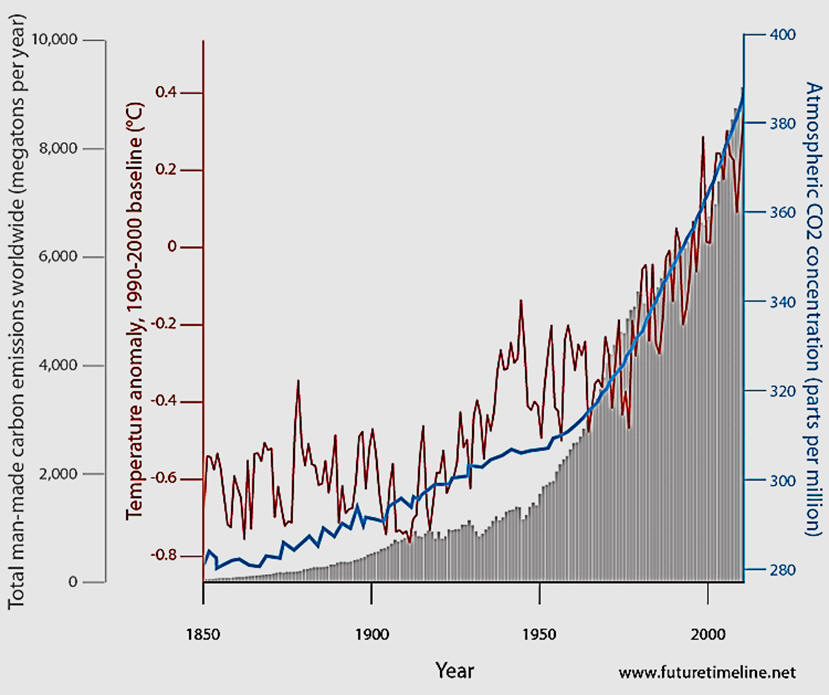
Many name carbon dioxide among the top three.
It is difficult to understand how people can be so opinionated on global climate change if they do not even know that carbon dioxide ranks number 4 but far down in concentration value from the top three. Because carbon dioxide is a minor constituent of the air, combustion of fossil fuels by humans has produced sufficient CO2 to increase its concentration from 280 ppm to over 410 ppm. [8]Even if people notice the units of ppm, most do not realize that these values translate to very low concentrations. While the consequences of the 46% increase in CO2 atmospheric concentration on the global climate are difficult to predict with high certainty, understanding that human activity has changed the content of the atmosphere does help when trying to explain the reason for concern. Unfortunately, scientific studies of the effects of increasing CO2 and other Greenhouse gases are consistent with an alarming increase in global air and water temperatures with severe consequences for life everywhere. For a Powerpoint presentation on climate change, please visit reference [8c]
pH is another measurement that does not receive sufficient attention with regard to interpretation of its values. When it is stated that neutral aqueous solutions have a pH of 7, how many think about how low the acid and base concentrations are in neutral water.
Q10. What is the pH of a solution prepared by the addition of 1 drop (0.05 mL) of 1 M acid to 1 L of pure water.
Q11. How much sodium chloride is needed to prepare 1 L of 1x10-7 M NaCl and is it possible to weigh the resulting quantity.
Q12. Why does the pH of freshly distilled water drop below a pH of 7 after sitting for a few hours open to the atmosphere?
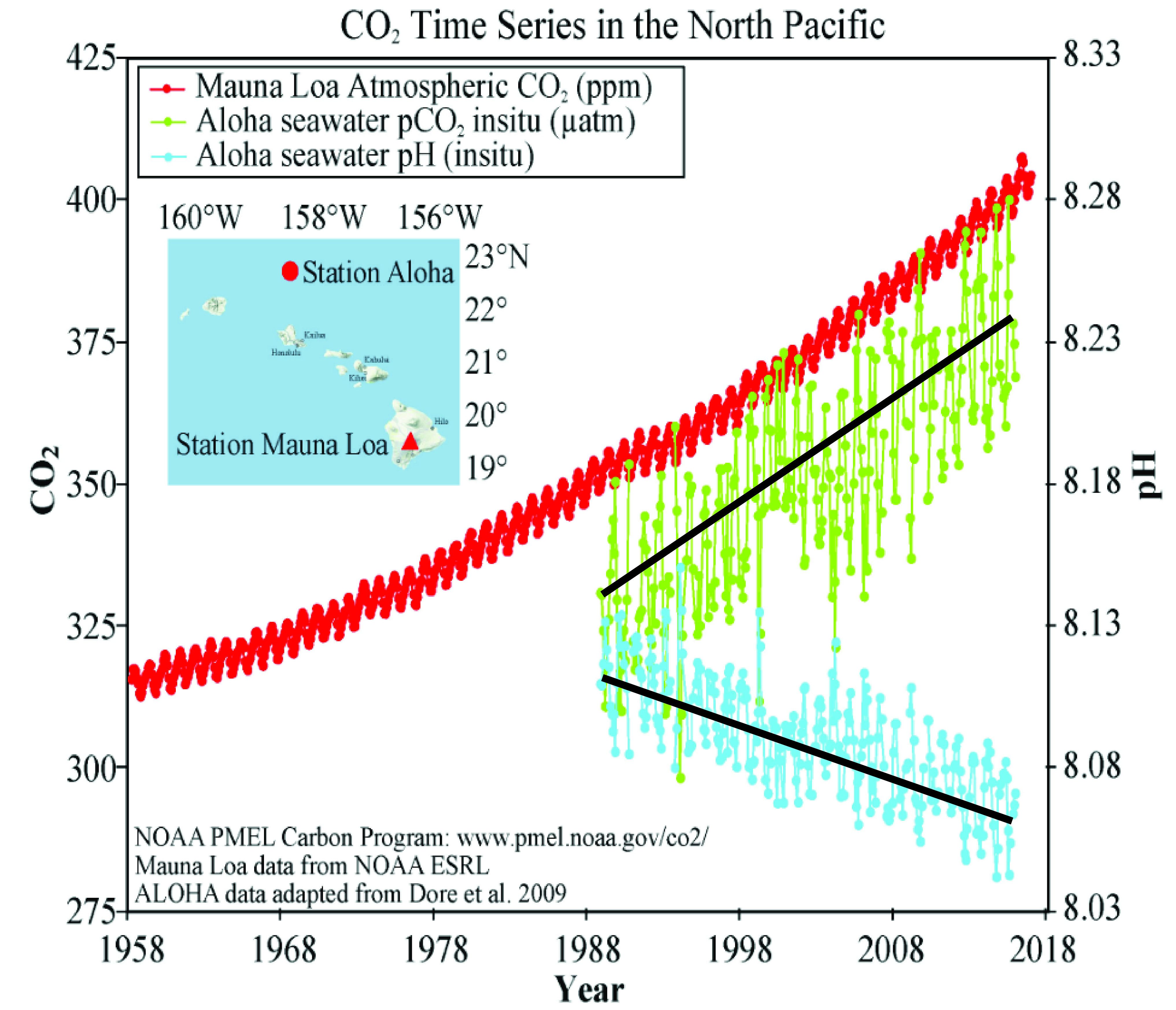
Strongly related to this concept is the observation that the pH of the oceans has decreased about 0.1 pH unit as a result of the atmospheric carbon dioxide increase and is threatening ocean life and the sustainability of coral reefs. [8] Thus it is possible to argue that because of ocean threats, air and water pollution, wars etc., even without consideration of climate change consequences, fossil fuels need to be phased out.
It is also important for students to think about the high end of practical concentrations. Most students have a real problem when asked to calculate the concentration of pure water (about 5M) and most will not have thought about the reason why acids generally do not and cannot exceed about 20 M.
Q13. What is the molarity of pure water (assume the density of water is 1 g/mL)?
Checking and correcting calculations.
For the final topic, the importance of
reflecting on the results of calculations, and learning from mistakes needs to be
emphasized.
Too often,
students obtain results and then move on without questioning the logic of the
result and its implications.
As a
carefully selected example, when students are asked to calculate the mass of an
antimony atom, some will come out with a result of 7.3x1025 g because
of the inverted use of Avogadro’s number.
Making this mistake is ok but it is not ok to
not notice that the answer is unreasonable as this value is close to the mass of the moon.
Calculations should always be followed
by the question:
Does the answer
make sense?
For example, values of
atomic and molecular mass have practical boundary values and answers out of the logical
range should lead to rechecking of the problem.
The simple technique of using insight to
determine if the result makes sense can frequently lead to a discovery of a
calculation error.
Instructors are often too lax when it comes to encouraging students to learn from
their mistakes.
Students need to be
encouraged and perhaps required to correct missed problems on tests.
Students have a tendency to merely look
at the score and not learn from their mistakes.
Probably more important than a tool for
assessing progress in a course, tests should function as learning
instruments.
Insight gained from missed problems can go a long way
toward making a student more competent in
chemistry.
When students realize they have acquired insight in
the field of chemistry, they will become the positive learners and will enjoy learning.
***********************************************************************************************************************************************************
ANSWERS TO QUESTIONS
Q1. O.N. of permanganate MnO4-, chromate CrO42-, chlorate ClO3-, perchlorate ClO4- . 7, 6, 5, 7, 6 Return to Q1.
b. BaSO4 and ZnSO4 The first
is insoluble in water and the second is soluble.
c. acetone and
hexane
The first is
soluble in water and the second is insoluble.
Return to Q6.
a. Because 1-pentanol is polar (with
potential H bonding), the intermolecular attractions are higher than in hexane.
b. Everything else being equal, boiling points generally correlate with
molecular mass due to greater V-D-W forces. However, water is not just
polar but also strongly hydrogen bonds to itself making its boiling point very
high. Compared to water, hydrogen iodide has a much higher molecular mass
but polarity is a much larger factor. The boiling point of HF is also
higher than that of HI due to greater intermolecular attractions.
Return to Q7.
Visitor counter started 11/28/20
unique visitors started 11/28/20
[2] Packer, J. E.; Woodgate, S. D., J. Chem. Ed., 1991, 68, 456.
[3] Dewit, D. G., J. Chem. Ed., 1994, 71, 750.
[4] Gordon Sproul https://pubs.acs.org/doi/10.1021/acsomega.0c00831
[5]
[6] Urbansky, E, T., Bioremediation Journal, Vol. 2, 2, 81-95. (1998)
[7] Rayner-Canham, G. and Huelin, S., Chem 13 News, Sept., 2000, #286, 6, 7.
[8]
a.
http://www.physicalgeography.net/fundamentals/7y.html
(accessed
b.
http://murov.info/climatechange.htm
(accessed
[9]
a.
http://www.pmel.noaa.gov/pubs/PDF/feel2899/feel2899.pdf
(accessed
b.
http://murov.info/climatechange.htm#Ocean
acidification, coral reefs (accessed