HIGHLIGHTS OF CHEMISTRY: A PERIODIC TABLE BASED APPROACH
This brief introduction to the basic concepts of
chemistry is intended as a starting point that hopefully will motivate you to expand your experience in chemistry. There are several
links at the end that provide substantially more coverage and detail.
For any science course, it is necessary to do more than just read text.
It is often stated that "one picture is worth a thousand words." Each of
the figures should help to illustrate a concept and the figures should be
carefully studied. Also, frequent links are provided to interactive multiple choice questions that were
designed for a traditional one semester basic chemistry course. To gain an
understanding of chemistry, it will be necessary to do problems as you read.
Hopefully you will find this site to be useful. Please send your comments
and suggestions to the e-mail address at the bottom.
For chemistry exercises with instant feedback, please visit:
http://murov.info/chemexe.htm
For information on periodic tables and ions, please visit:
http://murov.info/periodictables.htm
and http://murov.info/pertabtrends.htm
.

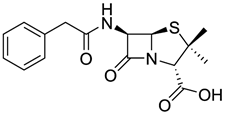
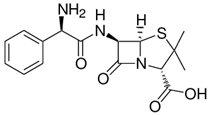
The serendipitous discovery that penicillin G kills bacteria by Alexander Fleming in 1928 eventually led to the development of the first antibiotic. Penicillin G significantly improved the ability of the medical field to successfully treat bacterial infections. It took several years and considerable research after Fleming's discovery to find methods to deliver penicillin G to humans. Unfortunately, penicillin G is destroyed by stomach acid and has to be injected. To overcome the problem, chemists were able to synthesize penicillin derivatives with a modification that stabilized the penicillin in acid. Incidentally, a method to introduce the NH2 group to provide acid stability is not a simple one step reaction and required substantial research, ingenuity, dedication, patience and time. The discovery, modification and application of penicillin is just one of the myriad of examples that illustrate how chemistry is used to improve the quality of our lives.
| penicillin G | ampicillin | amoxicillin | |
 |
 |
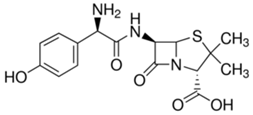 |
Please note that the significant difference between penicillin and its two improved modifications is the inclusion of a NH2 group that like ammonia (NH3) reacts with acid and stabilizes the molecule in acid medium. |
Chemistry is the study of the properties of matter and the energy changes that accompany transformations of matter. An alternate description is that chemistry is a search for order in matter. The periodic table of the elements is an exquisite result of the search for order in matter. Since all this stuff around us is a form of matter and chemistry connects all of the sciences, chemistry is often called the Central Science. To optimally navigate our way through our lives with the diverse chemically related challenges that confront us, it is vitally important that everyone be chemically literate. From the medicines, cleaning agents, fertilizers to energy sources like fossil fuels, a knowledge of the basics of chemistry will help you make the best decisions to improve the quality of life for yourself and society.
Many different approaches have been used to facilitate the learning of chemistry. From approaches based on history, the atom or the mole, chemistry presents challenges for all of us. However, the quality that is really required to learn the essence of chemistry is a positive attitude. The approach used here will be based on the Periodic Table of the Elements. This approach will emphasize the development of insight as contrasted with memorization. Through insight it is possible to enable understanding and the ability to apply the information to new situations.
 |
While many people such as the alchemists had attempted to utilize chemical reactions for personal gains, it was not until about 1778 that chemical experimentation could be said to be following the scientific method. In 1869 as a result primarily of the publication of the relative atomic masses of many elements by Berzelius and Cannizzaro (1860), Dimitri Mendeleev arranged the known elements in order of ascending atomic mass and in groups based on chemical behavior. The basic form illustrated above of the Mendeleev Periodic Table of the Elements remains today as the general format for the ordering of the elements. The Periodic Table will be used as the jumping off point for the learning of many concepts of chemistry. For a timeline on the development of the periodic table, see: http://murov.info/timelines.htm . |
 |
| Antoine Lavoisier | Lavoisier is considered by many as the founder of chemistry. He conducted quantitative experiments and proved that mass is conserved in chemical reactions (1778). | Dimitri Mendeleev |
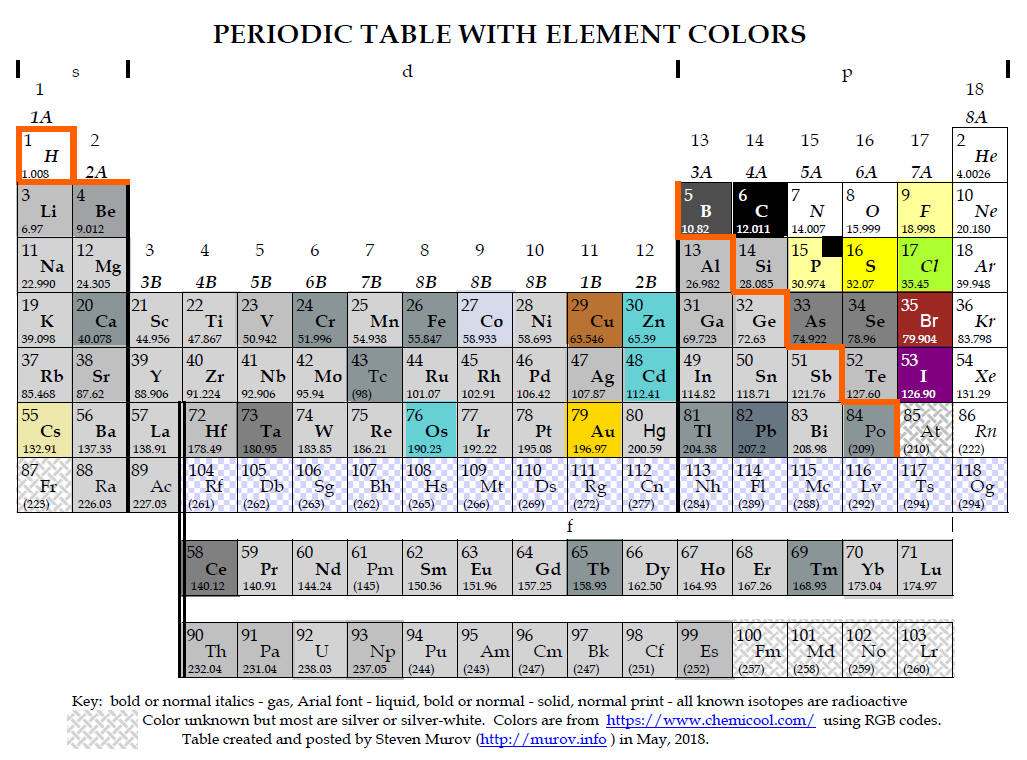
Knowledge has advanced a long way since Aristotle and Plato (about 340 BC) incorrectly claimed that matter is continuous (no discrete units called atoms) and accepted Empedocles (450 BC) claim that earth, air, fire and water are the four elements from which all matter could be made. Today, based on overwhelming evidence, we accept that all matter is composed of two types of substances, elements and compounds. Rather than being continuous, the elements are composed of discrete and chemically indivisible units called atoms. Compounds are composed of two or more elements making the atoms of elements the basic building blocks of matter as we know it. The table above contains the symbols for the 118 elements that have thus far been discovered. It should be noted that except for trace amounts of plutonium, none of the elements after uranium (#92) occur naturally on earth and numbers 93 through 118 have been made in the laboratory by nuclear chemists and physicists. Most of these transuranium elements have short half lives and will be discussed later under the topic of nuclear stability and radioactivity.
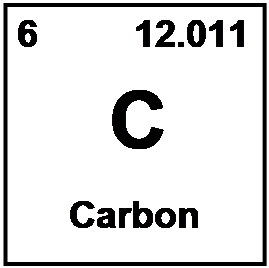
To begin this discussion. please focus on the box of the element, carbon, that is very important for the existence of life. Other than a few small and very important molecules like water and oxygen, molecules containing carbon are responsible for the lion's share of the structure and functioning of our physiology. Many periodic tables such as the example above for space reasons do not include the name of the element in the box. It is important to learn the symbols for many of the elements. As the symbol for carbon is C, carbon is one of the easy ones. However, others like sodium (Na), potassium (K), silver (Ag), have symbols derived from the Latin name of the element. The list of the 36 elements below comprise the minimum number of element - symbol relationships that need to be learned.
| atomic number | element | symbol | atomic number | element | symbol | atomic number | element | symbol | ||
| 1 2 3 6 7 8 9 11 12 13 14 15 |
hydrogen helium lithium carbon nitrogen oxygen fluorine sodium magnesium aluminum silicon phosphorous |
H He Li C N O F Na Mg Al Si P |
16 17 18 19 20 22 24 26 27 28 29 30 |
sulfur chlorine argon potassium calcium titanium chromium iron cobalt nickel copper zinc |
S Cl Ar K Ca Ti Cr Fe Co Ni Cu Zn |
35 47 50 53 60 74 78 79 80 82 92 94 |
bromine silver tin iodine neodymium tungsten platinum gold mercury lead uranium plutonium |
Br Ag Sn I Nd W Pt Au Hg Pb U Pu |
Although the concept of the atom was first proposed by Democritus and his mentor Leucippus around 440 BC, the concept of atoms was not taken seriously until 1803 when John Dalton, based on experimental evidence, reintroduced the concept that matter is made up of atoms. By the time that Mendeleev published his periodic table in 1869, the concepts of atoms as the basic unit of an element had generally been accepted but there was still no model for the composition of atoms. It was not known if atoms were totally unique for each element or whether atoms contained some common features.
| John Dalton''s atomic
model. 1. All matter is composed of extremely small particles called atoms. 2. Atoms of a given element are identical in size, mass, and other properties. (today we recognize that this is not accurate) Atoms of different elements differ in size, mass, and other properties. 3. 4. Atoms of different elements combine in simple whole number ratios to form chemical cmpds. 5. In chemical reactions, atoms are combined, separated, or rearranged. |
John Dalton |

|
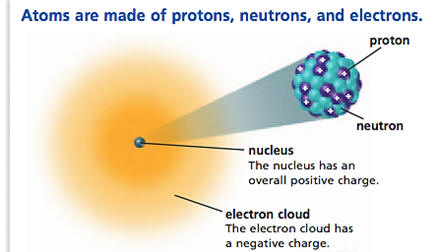
| Between 1869 and 1932, experiments demonstrated that all atoms are composed of protons, neutrons and electrons. It had also been demonstrated that most of the mass of the atom is concentrated is a small volume of the atom called the nucleus and that the nucleus is composed of protons and neutrons. For neutral atoms, a number of electrons equal to the number of protons occupy the space of the atom surrounding the nucleus. The number of protons in the nucleus determines the element and is called the atomic number. For the first 20 elements, the number of neutrons is approximately equal to the number of protons but after calcium (atomic number 20), for each additional proton, the number of neutrons increases on average by more than 1. As a result, by the time uranium is reached, there are 92 protons and 146 neutrons. |
 |
 |
 |
 |
 |
 |
| William Crookes | Eugen Goldstein | J. J. Thomson | Ernest Rutherford | James Chadwick |
| Crookes developed the Crookes tube which enabled Goldstein to discover the proton and Thomson to discover the electron. Rutherford experimentally discovered the nuclear models and Chadwick discovered the neutron. | Henry Moseley | |
| In the carbon box above,
the number 6 represents the atomic number of carbon and means that all
carbon nuclei contain 6 protons. An atom with any number different than
6 protons is a different element. The number 6 is an exact number as it
is not possible to have fractional amounts of protons, neutrons or
electrons. The number 6 determines the placement of the element in the
ascending order of elements. When Mendeleev used atomic mass to order
the elements, he recognized that there were some glitches but the proton
and atomic number were discovered several decades later. After the
discovery of the proton, Henry Moseley devised an experimental technique (1913) for determining the number of
protons in the elements. After this, the ascending order of the
elements in the periodic table was switched from atomic mass to atomic
number. Looking at the periodic table, what were some of the
glitches observed with use of atomic mass? |
 |
SCIENTIFIC NOTATION, MEASUREMENTS, SIGNIFICANT FIGURES, THE METRIC SYSTEM
Scientific
Notation. In the
sciences it is very common to encounter very small and very large numbers (e.g.,
the diameter of an atom is about 0.0000000001 m and 12 grams of carbon-12
contains 602,214,076,000,000,000,000,000 atoms =
6.02214076x1023 atoms). Because of the inconvenience of
writing such numbers and to avoid the confusion of significant figures in
numbers such as 320, scientists usually express numbers in scientific notation.
To use scientific notation:
1. Move the decimal point so that it becomes a number between one and ten:
63,000 becomes 6.3, 0.0035 becomes 3.5.
2. If, when the decimal point is moved, the resulting number, between one and
ten, is smaller than the original number (6.3 is smaller than 63,000) the
exponent will be the number of places the decimal was moved: 63,000 is
written 6.3x104 in scientific notation.
If, when the decimal point is moved, the resulting number, between one and ten,
is larger than the original number (3.5 is larger than 0.0035), the exponent
will be minus the number of places the decimal was moved: 0.0035 is
written 3.5x10-3 in scientific notation.
3. Whenever a decimal is moved, if the resulting number is larger than the
original, the exponent is made smaller by the same number of places.
0.012x10-5 = 1.2x10-7.
Whenever a decimal is moved and the resulting number is smaller than the
original, the exponent is made larger by the same number of places.
| The number 12.011 is called the atomic mass (or weight)
and represents the experimental value of the relative mass (compared to the mass
of other elements) of the element as it
is found on earth in amu (1 atomic mass unit = 1.66x10-24
grams). On other locations in the Universe, it is possible that
the mass will differ from the 12.011 amu found on Earth but the atomic number 6 for
carbon is universal.
On the amu scale, protons and neutrons have masses very close to 1
amu
(protons - 1.0073 amu, neutron 1.0087 -
amu). For reference, on this scale, an electron has a mass of about
0.000549 amu (atomic mass units) or 9.11x10-28
grams. Despite the huge differences between the mass of a proton and
electron, it is intriguing to note that protons and electrons have
charges equal in magnitude but opposite in sign. As expected from the
name, neutrons are neutral and do not have a
charge. It should also be noted that when together in a nucleus,
because of energy considerations, the mass values of protons and
neutrons is not
exactly additive. |
 |
|||||||||||||||||||||
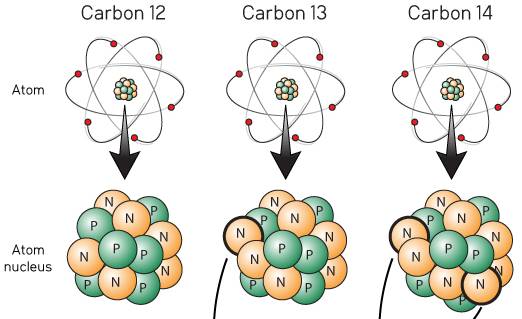
| Since carbon has 6 protons (with a mass of 6 amu) and an atomic mass of 12.011 amu, carbon on average must have 12.011 - 6 or 6.011 neutrons. The 0.011 is a result that can provide meaningful insight. 99% of carbon atoms on earth have 6 protons and 6 neutrons and by convention have an atomic mass of exactly 12. Although Dalton stated that all atoms of an element are identical, this statement has been modified. It has experimentally been determined that 1% of carbon atoms have 6 protons and 7 neutrons. Atoms with the same number of protons but different number of neutrons are called isotopes. Except for hydrogen, isotopes have very similar chemical and physical properties. Since there are two naturally occurring stable isotopes of carbon with 99% carbon-12 and 1% carbon-13, experiments yield an average mass of 12.011 for carbon (0.9890)(12.000 amu) + (0.0110)(13.003 amu). The figure to the right shows a third isotope of carbon with 6 protons and 8 neutrons. One part in a trillion of carbon is carbon-14. Because carbon-14 is radioactive with a half live of 5730 years, carbon-14 is often used to date plants and animals that died within the last 60,000 years. |
|
|||||||||||||||||||||
|
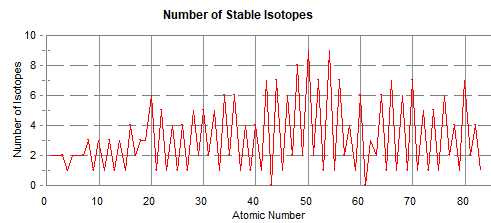
ISOTOPES AND ATOMIC MASS Although the periodic table does not directly provide information about isotopes, the atomic mass does give some significant information. The atomic mass in the table is an average of the isotopes that occur on earth. When the atomic mass is very close to a whole number, it is likely that there is a predominance of one isotope. There are a couple of exceptions such as bromine which coincidentally has two almost equal occurring isotopes, bromine-79 and bromine-81. When the atomic mass differs significantly from a whole number, there must be more than one naturally occurring isotope. For more information, an isotope table should be consulted. Chlorine with an atomic mass of 35.457 consists of about 75% chlorine 35 and 25 % chlorine 37. In a later section on nuclear chemistry, the proton - neutron ratio and the effect of the ratio on the stability of the nucleus will be briefly discussed. Notice to the right that for odd atomic numbers, there is commonly only one or two stable isotopes. For even atomic numbers, there are often many stable isotopes. |
 |
For some exercises on significant figures, please
visit
http://exercises.murov.info/ex3-2.htm
and do numbers
1-27, 30, 31, 33.
Measurements such as the experimental determination of atomic mass are limited by the precision of the techniques used for performing the measurement. For carbon, it has been possible to determine the mass to 4 digits beyond the decimal or 12.0107 amu. For convenience, it has been rounded off here to 12.011 amu. This means that the measurement has high confidence in the first three digits after the decimal but that the last digit (7) measured many times would average 7 but the measurements would vary between 6 and 8. To clarify the precision of a number, a convention called significant figures has been developed to indicate the precision of a measurement.
Suppose that a leg on a table has broken and needs
to be replaced. Measurement of the other 3 legs reveals that the legs are
70 cm long. it must be stressed
that the units of a measurement are just as important as the number itself.
If the units are not included and you assume that the legs are 70 inches, the
table leg you make will be about 2.5 times as long as required and not useable.
Mistakes like this are unfortunately not completely uncommon. A passenger flight to Edmonton (Flight
143) from Toronto based the calculation of the amount of fuel needed on liters when the
number actually associated with the amount needed was in gallons. The plane ran out of
fuel but glided to a safe landing near Winnipeg far short of its intended
destination. Also a Mars lander crashed into Mars because of a
misunderstanding of the units involved.
For some exercises on this flight event, visit
http://exercises.murov.info/ex2-1.htm
and do problems 14-16.
When you are told the table leg
length should be cut to 70 cm, does this adequately give you all the information
you need about the length of
the leg? In other words, does 70 cm tell you how careful you have to be when you
measure and cut the table leg. If you assume that 70 cm means between 65 cm an 75 cm, it is likely
that the table will end up with a huge wobble. Even if you assume
that the leg should be between 69.5 cm and 70.5 cm, a bad wobble is likely
unless you are really lucky. If you assume that the leg should be between
69.95 cm and 70.05 cm the leg should come out to be an acceptable length and the table should
not have a wobble. If you decide to make
the length between 69.995 cm and 70.005 cm, you probably will spend too much
time trying to achieve this especially if you are cutting wood on a table saw.
Thus, how does one communicate that the leg should be between 69.95 cm and 70.05
cm? Saying 70 cm leaves the door open to all the above options but probably
indicates accuracy is not important when it clearly is. Scientists have
clarified this issue by establishing the concept of significant figures.
Significant figures afford an easy method for communicating the precision
needed without a more complex calculation of the tolerance. In this case, measuring and
cutting tools should be used that will provide a leg between 69.95 cm and 70.05
cm. The length of the table should be given to 3 significant figures or
70.0 cm so that the leg will come out between 69.95 cm and 70.05 cm.
To summarize, if the value 70 cm is the desired length, use of significant
figures means between 65 cm an 75 cm. If
7.0x101 (or 70.) cm is specified, then the leg will come out the still unacceptable
69.5 cm to 70.5 cm. For the desirable 69.95 cm to 70.05 cm, the length
should be specified as 7.00x101cm. A specified value of 7.000x101cm
will be very difficult to achieve and probably a waste of time. It should
be noted that determining the number of significant figures is straightforward
unless there are zeros present. If there is no decimal showing count from
right to left from the first non-zero digit. If a decimal is showing,
count from left to right from the first non-zero digit.
| example | number of significant figures | example | number of significant figures |
| 3.14 | 3 | 5281 | 4 |
| 3.140 | 4 | 5280 | 3 |
| 3.040 | 4 | 5280.0 | 5 |
| 0.14 | 2 | 5280.00 | 6 |
| 0.140 | 3 | 5.28x103 | 3 |
| 0.0140 | 3 | 5.280x103 | 4 |
| 0.0001 | 1 | 0005 | 1 |
Some of you probably do not have a sense for the centimeter as a unit of length. There are many advantages of the metric system over the U.S. customary system. Even though there would be a significant cost for the U.S. to switch to the metric system, in the long run it would be easier and cheaper if the U.S. switched. Try to gain some experience with the metric system so that you can think metrically instead of having to convert.
1. Units are related by powers of 10 rather
than by factors of 3, 12, 16, 36, 5280 etc., making it much easier to convert.
2.
Volume units are defined by distance cubed thus 1 mL exactly equals 1 cm3.
3.
The density of water is about 1 g/mL at working temperatures (0.998 g/mL at
20oC) and exactly 1 g/mL at 3.98oC. In the U.S., it is stated
that a pint is a pound the world around but this has a 4% error.
4.
The metric system is used by most countries and it would be much better if only
one set of tools could be needed.
5.
The metric system has no need for mixed units such as 5 ft, 7 inches or 8 lbs, 6
oz.
6.
Abbreviations in the metric system are logical as contrasted with pound = lb and
ounce = oz.
Examples:
| Item | Mass | Item | Volume | Item | Distance | Item | Temperature (oC) | |||||
| penny | 2.5 g | 1 drop | 0.05 mL | penny diameter | 1.9 cm | absolute zero | -273.15 | |||||
| hot dog | 45 g | cup of coffee | 120 ml | soda can diameter | 6.6 cm | bp of liquid nitrogen | -196 | |||||
| golf ball | 46 g | soda can | 355 mL | baseball diameter | 9.4 cm | mp of water | 0 | |||||
| banana | 120 g | 1 qt | 0.946 L | soda can height | 12.2 cm | normal human body | 37 | |||||
| baseball | 150 g | gallon of gasoline | 3.79 L | basketball diameter | 24.3 cm | room temperature | 21 | |||||
| head of lettuce | 800 g | bathtub | 300 L | table height | 70 cm | bp of water | 100 | |||||
| baby | 3 kg | ceiling height (8 ft) | 2.44 m | |||||||||
| adult | 45 - 135 kg | Los Angeles to N.Y. | 3944 km |
For some exercises on significant figures, please visit http://exercises.murov.info/ex1-1.htm and do numbers 1-15.
Percent by mass of some elements of the 24 most abundant elements in the
universe.
(for more data, see:
https://periodictable.com/Properties/A/UniverseAbundance.html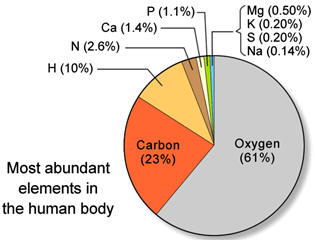
| element | universe | solar system | earth crust |
human | element | universe | earth crust |
human | ||||
| hydrogen | 73.9 | 70.6 | 0.15 | 10.0* | nickel | 0.006 | 0.009 | 1x10-5 | ||||
| helium | 24.0 | 27.5 | 6x10-7 | aluminum | 0.005 | 8.1 | 9x10-5 | |||||
| oxygen | 1.04 | 0.96 | 46.6 | 61 | sodium | 0.002 | 2.3 | 0.14 | ||||
| carbon | 0.46 | 0.30 | 0.2 | 23 | chromium | 0.002 | 0.01 | 3x10-6 | ||||
| neon | 0.14 | 0.15 | 3x10-7 | manganese | 0.0008 | 0.11 | 2x10-5 | |||||
| iron | 0.10 | 0.12 | 6.3 | 0.006 | phosphorous | 0.0007 | 0.1 | 1.1 | ||||
| nitrogen | 0.096 | 0.11 | 0.002 | 2.6 | cobalt | 0.0003 | 0.003 | 2x10-6 | ||||
| silicon | 0.07 | 0.06 | 27 | 1.4 | titanium | 0.0003 | 0.7 | 1x10-7 | ||||
| magnesium | 0.06 | 0.05 | 2.9 | 0.5 | potassium | 0.0003 | 1.5 | 0.2 | ||||
| sulfur | 0.05 | 0.04 | 0.04 | 0.2 | vanadium | 0.0001 | 0.02 | 3x10-6 | ||||
| argon | 0.02 | 2x10-4 | chlorine | 0.0001 | 0.02 | 0.12 | ||||||
| calcium | 0.007 | 5 | 1.4 | fluorine | 0.00004 | 0.05 | 4x10-3 | |||||
| *atom percent of hydrogen is 62% |
Although 118 elements have been discovered, 91% of the estimated 1080 (1 with 80 zeros after it) atoms in the universe are atoms of the simplest element, hydrogen and most of the rest are helium. Since hydrogen is the lightest element (mass of 1 amu), by mass, the percent of hydrogen is a lower number or 73.9%. 99.985% by number of hydrogen atoms have 1 proton and 1 electron. A second stable hydrogen isotope with one neutron (deuterium or heavy hydrogen) is only 0.015% (0.031% by mass) of naturally occurring hydrogen. This is one of the few isotopes with its own name, deuterium or heavy hydrogen. In stars, the energy provided by the nuclear fusion of hydrogen-1 into helium is the reason that helium is the number 2 element in abundance in the universe. Fusion in stars such as our sun provides the energy that enables life to exist on earth. Additional fusion reactions produce elements up through element 26 (iron). Production of elements with more protons than iron need an even more energetic source than nuclear fusion in stars such as the conditions in a super nova. After a star goes through its life cycle and terminates, the star remnants sometimes end up being part of a new star and its solar system. Since our earth has 90 naturally occurring elements, this means that our sun and its planets comprise a second generation system. The elements that make up the earth and our human bodies were formed in a previous star and from a cataclysmic astronomical event.
| With the addition of protons to the nucleus, we progress to the other elements in addition to hydrogen that are vital to the existence of life. Carbon, nitrogen and oxygen as well as sodium, magnesium, phosphorus, sulfur, potassium, iron and others have crucial roles in plants and animals. As stated, all substances are either elements or compounds. Since most elements have a tendency to react with other elements including the ubiquitous oxygen, most substances encountered on earth are compounds, Only a few elements are found in elemental or native form on earth including the elements with the smiley faces. Because the inert gases are non-reactive, they are found only in elemental form. Some of the metals such as silver, platinum and gold are sometimes called inert or precious metals because of their low reactivity. Because of the low reactivity and their pleasing luster, these metals are commonly used in jewelry. |
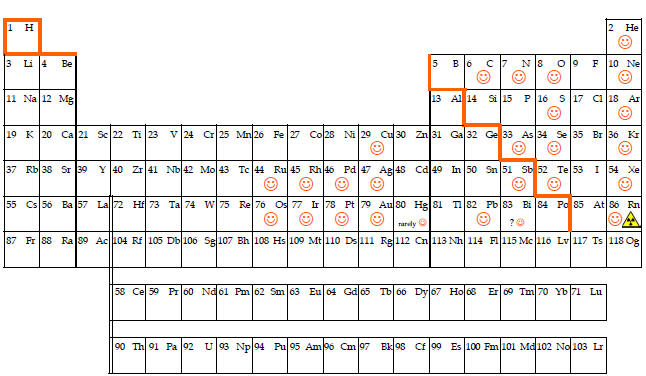 |
The artificial orange line that appears to be a staircase is commonly included in periodic tables to divide the metals from the non-metals. Except for mercury (and hydrogen which is marginally described as having metallic properties), metals are solids that are malleable, ductile, conduct heat and electricity and are lustrous. Non-metals include gases, solids and a liquid and are generally non-conductors or insulators and brittle. Despite the inserted orange staircase, there is no sharp divide between metals and non-metals and the elements touching or near the staircase are often called metalloids with properties intermediate between metals and non-metals. Some appropriately play an important role in electronic technology as semiconductors. Please note that the periodic table at the top presents the approximate colors of the elements and provides another way for distinguishing metals and non-metals with most metals having silver or gray like color and the non-metals colorless or varied colors.
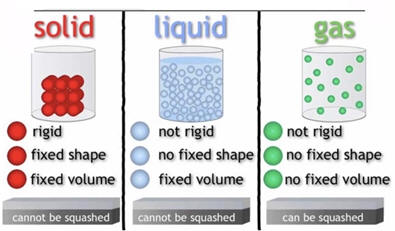
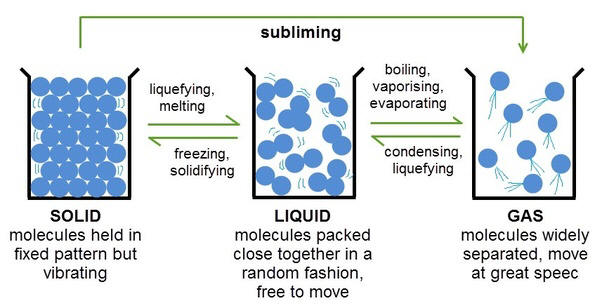
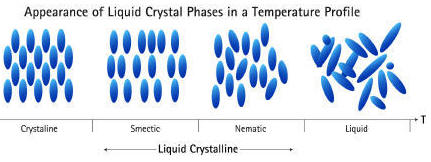
States of Matter. To gain a better understanding of the elements, we need to explore their physical and chemical properties. It is sometimes said that there are three states of matter: solid, liquid and gas. For the sake of accuracy, the statement could say there are three common states of matter but even the addition of the word common leaves much to be desired. Most of the matter visible to us is the matter in stars which is a fourth state of matter called a plasma. The distinction between a plasma and our three common states of matter is that stars are so energetic that the electrons move independently of the nuclei as contrasted with the substances around us that have electrons intimately associated with nuclei. Actually, today it is common to encounter a 5th state of matter called LCD or liquid crystal display as the display on many electronic devices and some digital watches. The liquid crystal state exists only for a selected number of substances and has properties intermediate between a solid and liquid. The unusual properties of an LCD make them ideal for many applications.
Of the 118 elements most are solids, about 11 are gases (most are the colorless
squares in the periodic table above + fluorine and chlorine) and only mercury
and bromine are liquid under average conditions on earth. Gallium and
cesium do melt and become liquids on very hot days.
For some exercises on significant figures, please
visit
http://exercises.murov.info/ex3-1.htm
and do numbers 4,
7, 9, 15 20, 26, 35, 37, 42.
 |
 |
 |
Physical Properties (properties that can be determined without a chemical change) are determined for at least two very important reasons. After an unknown substance is discovered or synthesized, measurement and recording of physical properties enables researchers to identify the substance the next time it is encountered. Second, the very important field of material science often involves the search for substances with certain desirable properties. Physical properties should be straightforward to measure and fall into two categories. Extrinsic physical properties depend on the amount of the material such as mass or volume and are not useful for identification purposes. However, melting and boiling points, density, and refractive index are the easiest of the intrinsic physical properties to measure and often enable the identification of an unknown substance.
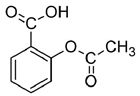
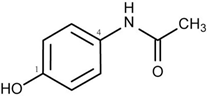
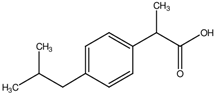
Suppose that the label of a bottle that contained one of the analgesics, aspirin, acetaminophen, ibuprofen or naproxen has fallen off.
| substance | acetyl salicylic acid (aspirin) | acetaminophen (e.g., Tylenol) | ibuprofen (e.g., Motrin) | naproxen (e.g., Alleve) | |
| melting point | 140oC | 169oC | 78oC | 153oC | |
| molecular structure |
 |
 |
 |
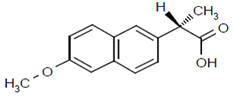 |
Melting points or the temperature at which the solid phase liquefies are easily measured on very small amounts of sample. Since melting points are measureable to a couple of degrees, determination of the melting point should distinguish among the four possibilities. It is even possible to verify the identity using melting points if authentic samples of the possible analgesics is available. Impurties lower and broaden melting points. By mixing the unknown with a small amount of the known and repeating the melting point measurement, two possible observations are possible. A broadened and depressed melting range indicates the two substances are different but if the two match, the melting point will be the same as previously measured. Impurities broaden and lower melting ranges. It should be noted that melting points for commercially available analgesics will not be straightforward as the tablets contain binders to enable molding of a pill.
As an example of the considerations in material science, suppose a metal needs to be selected for a rocket engine part and the options available are in the table below. The high melting point and relatively low density might lead you to favor titanium. In addition, titanium has the additional possibly valuable property that its properties are less sensitive to temperature changes as indicated by the thermal coefficient of expansion. Titanium abundance might raise questions about cost and indeed the cost for even low grade titanium is much higher than for the other three metals. However, abundance is not the main problem as titanium compounds are readily available as illustrated by the use of titanium oxide as the white pigment in paint. Early on, aluminum was much more expensive than today because like the other elements, it is found in nature combined with other elements. Breakthroughs in the refining process for aluminum lowered its price to a competitive level. The refining process for titanium remains very costly. If you can develop an inexpensive refining process for titanium, undoubtedly, you will very quickly find yourself rich and surrounded by many more products that contain titanium. For a rocket it is possible that despite the cost, titanium could be the metal of choice. For other applications where the melting point is not important, because of low density and much lower cost, aluminum might be the choice. If strength is the most important consideration, then steel could be the choice but another consideration is reaction with oxygen to give rust. Titanium and aluminum are much more resistant to reaction with oxygen.
| substance | melting point (oC) | density (g/cm3) | tensile strength | thermal coefficient of expansion (10-6m/(m K)) |
abundance in earth's crust (%) |
price ($/kg) | ||
| aluminum | 660 | 2.708 | 310 | 22 | 8.1 | 1.51 | ||
| titanium | 1670 | 4.506 | 434 | 8.5 | 0.7 | >17 | ||
| iron | 1538 | 7.874 | 540 | 12 | 6.3 | 1.21 | ||
| steel | 1370* | 8.05* | 420* | 13* | NA | 0.60* | ||
| *depends on alloy |
For some exercises on states and properties of matter, please visit http://exercises.murov.info/ex2-2.htm and do numbers 1, 5, 6, 8, 9, 14, 15, 16, 21, 23, 30, 34, 39.
COMPOUNDS, PERIODICITY OF FORMULAS
| The
alchemists had a least two goals: finding the philosophers stone (or
the secret to longevity) and the ability to convert lead into gold. The
latter is an interesting and intriguing goal. But, unknown to the
alchemists, it is impossible to achieve using chemical reactions.
The image to the right is an engraving by the French artist
Pierre-Francois Basan (1723-1797), after a painting by Flemish artist
David Teniers II (1610-1690). The translation of the title is
The Pleasure of Fools. Teniers was parodying an early similar
alchemist painting but he replaced the human chemist with an ape.
Alchemists probably could have been appropriately called foolish but
today the opposite is true of chemistry. An appropriate
description of chemistry would be one of the pleasures of the caring.
Those that care will want to acquire sufficient chemistry literacy to
enable the best decisions to be made on many critical issues such as
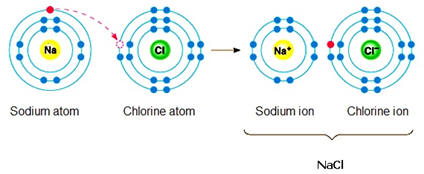
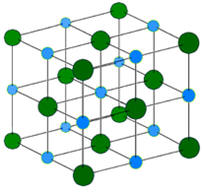
medicines, climate change, use of plastics and pollution. Today we do know that it should be possible to convert lead into gold using nuclear but not chemical reactions. However, the cost of this process would likely be much more than the value of gold produced. Chemical reactions involve primarily changes in electron characteristics. If a sodium atom were to encounter a chlorine atom, there is a real possibility that an electron will jump from the sodium atom to the chlorine atom. This results in the formation of a positively charged sodium ion or cation as the sodium will now have one more proton than the number of electrons. Correspondingly, the chlorine having acquired an extra electron will become a negatively charged ion or anion. Since positively charged species are strongly attracted to negatively charged species, the sodium cation and chlorine anion (chloride) will form a very strong ionic bond with the formation of the compound sodium chloride (table salt). Please note this is a huge oversimplification as in a sodium chloride crystal, each sodium is surrounded by several chlorides and vice versa. |
 |
 |
 |
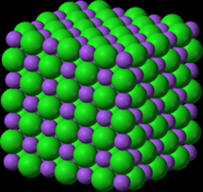  |
Formulas of compounds with hydrogen and chlorine.
The Periodic Table below
contains the formulas of many compounds of the elements when bonded to hydrogen
or chlorine. The reaction above between sodium and chlorine is
hypothetical. Sodium chloride in oceans was probably formed via a complex
sequence of reactions rather than a direct reaction between two elements that
are extremely reactive and never found in nature in elemental form. The important
observation below is the ratio of the cation to anion in the formula (e.g. for
NaCl - 1:1, MgCl2 - 1:2, AlCl3 - 1:3, CCl4 -
1:3, NCl3- 1:3). When the elements combine, for compounds, the
net charge must be neutral. When sodium bonds, it always loses one
electron but when magnesium bonds, it always loses 2 electrons, aluminum loses 3
and carbon shares 4. Chlorine generally wants to gain one electron so
sodium and chlorine bond 1:1 as NaCl.
However, since magnesium donates 2 electrons, it takes 2 chlorines to
form a neutral molecule or MgCl2. Aluminum and chlorine
correspondingly form AlCl3. For slightly more complex cases
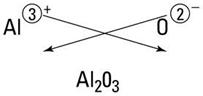
such as aluminum which donates 3 electrons and oxygen which seeks to gain 2, the
result is Al2O3 as 6 is the common factor. One
technique that can usually be used to deduce the formula is to imagine the
charge as a superscript after each partner and crisscross the charges to arrive
at the formula. Sometimes it is necessary subsequently to reduce the
subscripts to the lowest common denominator. Examine the formulas of the
compounds particularly for columns IA, 2A and 3A and proceed to the bottom of
the table.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| 1A | 1B | 3B | 4B | 5B | 6B | 7B | 8B | 8B | 8B | 1B | 2B | 3A | 4A | 5A | 6A | 7A | 8A |
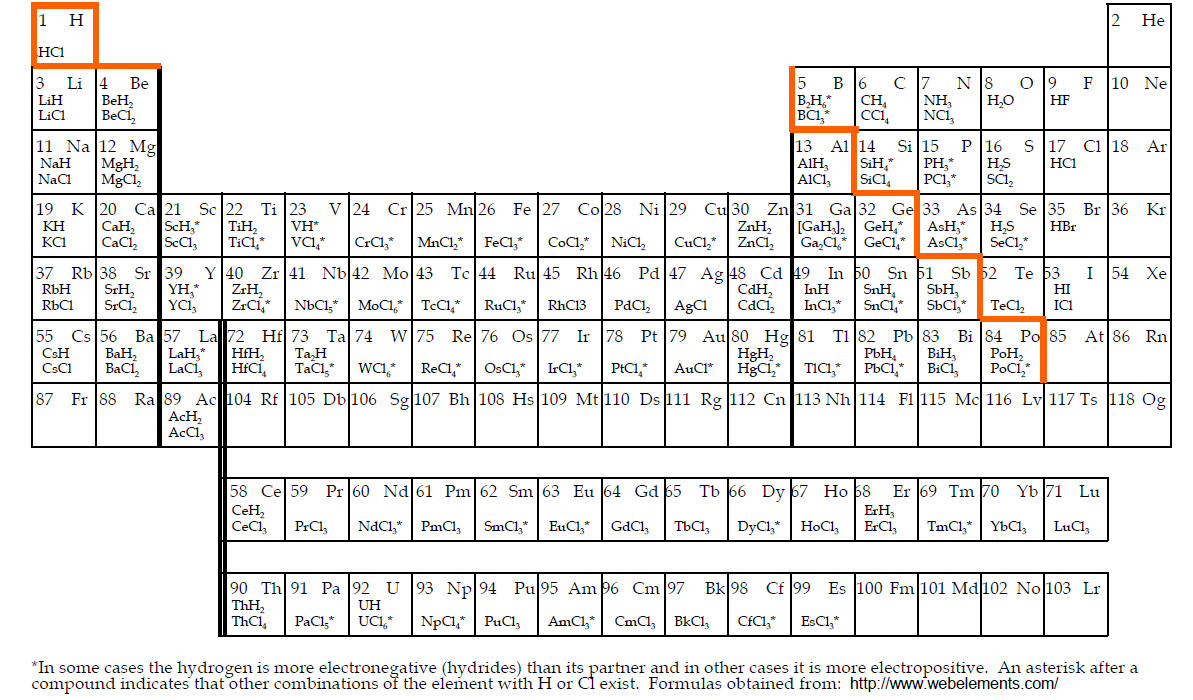
Earlier, it was mentioned that Mendeleev arranged the elements in order of increasing atomic mass (today atomic number) and arranged the format so that elements with similar properties were aligned in vertical columns called groups or families. For example, after fluorine (the inert gases were not discovered until about 30 years after Mendeleev published his Periodic Table), Mendeleev place sodium under lithium because of similar chemical reactivity. He found that the elements following sodium also have properties similar to the element above them until he reached chlorine. Potassium has similar reactivity to sodium and calcium resembles magnesium so another new period was started. The next 10 elements are transition metals and involve a discussion of electronic orbitals that is beyond the scope of this introduction. Resuming with the placing of gallium under aluminum lines up the properties across the rest of the chart. This process repeats for the rest of the chart. It is possible to observe this periodicity by noting that the formulas of the compounds in the columns tend to be the same but different from the adjacent column. A similar pattern is also found for the formulas of the compounds with hydrogen. However, hydrogen is more complicated because in some compounds it gives up an electron and in others it gains an electron. Note the formulas for the 6A and 7A elements combined with hydrogen. Generally they are 2:1 and 1:1 as the hydrogen gives up one electron and the 6A elements need 2 and the 7A elements need just 1.
Two sets of group numbers have been given above the chart. The top 1 through 18 is the international system but the bottom one with A's and B's is the American system. For use here, the American system provides more information. For elements in groups 1A - 3A, there is a strong tendency to give up the number of electrons designated by the group number. For elements in groups 6A and 7A, there is a tendency to gain 8 minus the group number of electrons. For elements in 4 A and 5A, most compounds in the first 3 periods form covalent (shared instead of transferred or ionic) bonds. These electron tendencies are intimately connected to the resulting formulas and chemical reactivities of each group and are an important part of the reason for the layout of the periodic table. A better understanding of the reason for the correlation of the group numbers with formulas is beyond the scope of this introduction but suffice it to say that electrons are describable with quantum numbers that provide a theoretical basis for the format of the periodic table. One conclusion is that energy and stability considerations favor electron orbitals that are full. For the Group A elements, the inert gases (group 8A) have filled orbitals and this is the reason they are inert. As a result, atoms in the groups 1A, 2A and 3A tend to lose 1, 2, or 3 electrons so that they will have the same number of electrons as the previous inert gas. Atoms in 6A and 7A tend to gain 2 or 1 electrons to advance their number of electrons to the next inert gas. Atoms in the middle such as carbon would have to give up or gain 4 electrons which results in a charge of +4 or -4. This high charge is too concentrated and instead of giving up or accepting electrons, carbon forms covalent bonds (shares electrons) to achieve an inert gas structure of electrons. The electron behavior of group B elements or transition metals is more complex but suffice it to say that most commonly they give up 2 or 3 electrons.
| You might have noticed that when including oxygen in a chemical reaction, the oxygen is written as O2 rather than O. When possible in reactions, chemists try to accurately represent the formulas of the chemicals. Several commonly encountered elements occur as diatomic molecules rather than as isolated atoms. Among these are hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine and iodine. These elements in atomic form are all extremely reactive and reaction to achieve an electron configuration of an inert gas is energetically favorable. Lacking other nearby opportunities, these atoms combine with themselves to share electrons and thereby achieve a pseudo inert gas electron configuration. However, better than sharing for these atoms is theft and these elements even as diatomics are still very reactive so that they can achieve even better possession of their outer electrons. That is one of the reasons that chlorine, bromine and iodine kill bacteria. Please note that nitrogen is somewhat exceptional in this quest as N2 has a triple bond and is fairly unreactive. This is fortunate as nitrogen is 78% or our air and its inertness is a good circumstance except that it makes nitrogen compounds difficult to make. |  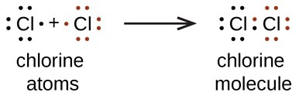 |
| When an element accepts electrons from its partner (e.g., Cl-), the ending of the name of the element is usually changed to ide (e.g., chlorine to chloride, oxygen to oxide). In addition to the negative ions of the elements, there are many groups of atoms that contain 1 - 3 extra electrons that bond together to form polyatomic ions. These ions tend to stay in tact during many chemical reactions and should be learned. A list of many commonly encountered ions and polyatomic ions is available at: http://murov.info/ionform.htm . The most common polyatomic ions are hydroxide - OH-, nitrate - NO3-, carbonate - CO32- and sulfate- SO42-. One very common positively charged polyatomic ion is ammonium - NH4+. Formulas can be determined using the criss cross method given previously [e.g. sodium carbonate - Na2CO3, ammonium sulfate - (NH4)2SO4]. Note the parentheses required when more than one polyatomic ion is needed to balance the charge. | 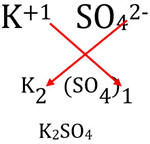 |
For
some exercises on polyatomic ions, please visit
http://exercises.murov.info/ex4-1.htm
and do numbers 1-10, 21, 24, 28, 30, 36, 38.
For some exercises on nomenclature, please visit
http://exercises.murov.info/ex4-2.htm and do numbers
1-10, 13-15.
For some exercises on
nomenclature, please visit
http://exercises.murov.info/ex4-3.htm and do numbers
1, 8, 9, 11,13..
At the heart of chemistry are the chemical reactions that enable all life and provide pathways for the synthesis of almost all of the materials that surround us. Synthetic chemicals make it possible to defeat diseases, enhance agriculture, make inexpensive and very light but strong products, clean everything from ourselves to the surgical theater and paint masterpieces. As with any product, chemicals such as drugs, pesticides and fossil fuels can be and are abused and overused throughout our environment. No chemical product should be used before both short and long term studies are done on safety and environmental impacts. The pros and cons of chemicals will be left to other sources as the purpose here is to introduce chemistry and provide literacy in chemistry. Complicating this section on chemical reactions is the very broad scope of the topic. Generally, to study a subject it is helpful to subdivide the subject into smaller but manageable categories. Many different ways to classify chemical reactions have been put forward but these classification systems because of the vast complexity of chemistry, all have significant limitations.
One very important point needs to be stressed with
regard to chemical reactions. Just because a reaction can be written does
not mean that the reaction will go left to right and if it does, at what rate
will it go. For example, without having additional information for the
reaction A + B = C + D it is equally
probable that the reaction could proceed left to right or right to left.
The information needed to determine this is usually a part of a study of the
thermodynamics and kinetics of the system. For the most part, a discussion
of the energetics involved in the reactions is above the level of this
introduction.
Classification systems for reactions.
Endothermic or Exothermic: Every reaction either consumes or gives off energy. Energy comes in different forms and this type of classification is useful when considering the conditions needed for carrying out the reaction but is not very useful as a descriptive method for classifying reactions.
Oxidation-Reduction or not Redox. Redox reactions involve transfer of electrons. This is a useful classification system but very broad.
Reaction Description: Decomposition, Combination, Combustion, Single Replacement, Double Replacement. This system works well in general chemistry courses but leaves out many types of reactions. Any combination reaction written in the reverse direction is a decomposition reaction.
Organic or Inorganic: There are many types of organic and inorganic reactions that do not conveniently fit into the categories immediately above. Organic chemistry has it own system of classifying reactions that encompasses many categories of reactions. The field of organic chemistry will be briefly discussed in a later section.
| There are some overlaps in the above classifications. All single replacement reactions are also redox reactions because single replacements include an element in its elemental state. An element cannot react without losing or gaining electrons. All combustion reactions are also redox reactions. Double replacements are never redox reactions. |
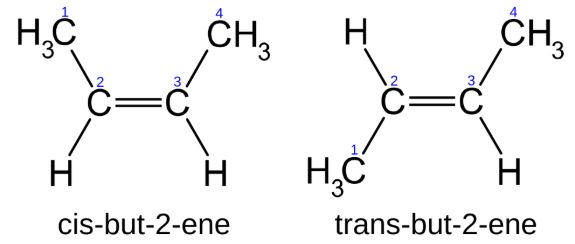 |
| Generally reactions involve transfer of electrons and/or breaking and formation of new bonds. As an example of the difficulties in classifying reactions, consider the reaction of cis-but-2-ene to trans-but-2-ene (top right). At first glance, there might be a temptation to conclude this is not a reaction as the sequence of bonds is the same for both molecules. However, those that know some organic chemistry recognize that these two molecules are geometrically different, do not interconvert at room temperature and have different physical and chemical properties. While there is essentially free rotation around single bonds, rotation around double bonds does not occur at room temperature. Which classification system best describes this isomerization reaction? It is slightly exothermic, not a redox, does not conveniently fit any of the Reaction Descriptions and it is an organic reaction. For this reaction to occur, one of the two bonds that make up the double bond must break followed by rotation and reformation of the bond but with the atoms in a new geometry. While this reaction does not occur at room temperature, input of energy in the form of heat or light can cause the reaction to occur. Why, you should be asking was this isomerization chosen as the first example? First, it is a very simple looking reaction that illustrates the difficulties of categorizing reactions. More importantly, if you observe the reaction of cis-retinal, you will see that it too involves an energy induced rotation around a double bond. In fact, the reason you can read these sentences is that light impacting your eye causes the reaction that sends a signal to your brain and enables your vision. | 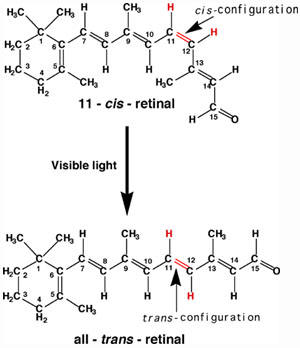 |
| Before leaving the butene reaction that can be described as a geometric change, lets consider another concept that the reaction illustrates. It was mentioned that the reaction is exothermic (gives off energy) but that it does not occur at room temperature. There are two general energy considerations in chemical reactions: the overall energy change (- 4 kJ/mol) and the energy changes that occur during the reaction. For the reaction to occur, the double bond needs to break and this requires energy. When the bond is reformed, the expended energy and more is regained in this case but energy input is required to break the bond. The magnitude of the energy required to break the bond is sometimes called the activation energy. The overall energy change determines if the reaction is capable of proceeding without continuous energy input but the activation energy determines how fast the reaction will occur. For the geometric isomerizations above, the reaction rate is so slow at room temperature that reaction is not noticeable. Heat or light considerably increase the rate of reaction. As a questionable analogy, imagine you are in Reno (altitude 4500 ft.) and want to go to Sacramento (altitude 30 ft.). While this is clearly downhill over all, it is not possible because of the mountain in between to coast from Reno to Sacramento. If you use enough energy perhaps in the form of gasoline, you can drive up and go over the Donner Pass and then proceed downhill (with several bumps in between) or a tunnel could be dug through the mountain to lower the height of the barrier. Digging a tunnel would be like adding a catalyst to increase the rate of reaction by lowering the barrier. As a very approximate guideline for unimolecular reactions such as this one, a barrier of more than 100 kJ/mole is required for stability or the molecule will not survive in a bottle for very long. | 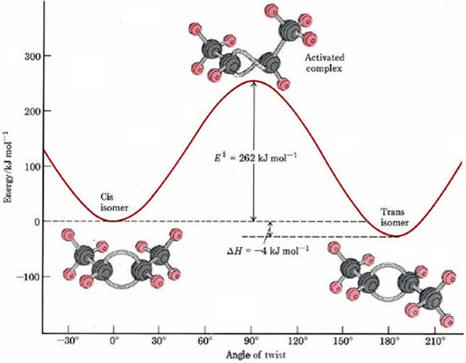 |
|
OH-
+
CH3Cl
=
CH3OH
+
Cl- Hydroxide
+
Chloromethane
yields
Methanol
+ Chloride |
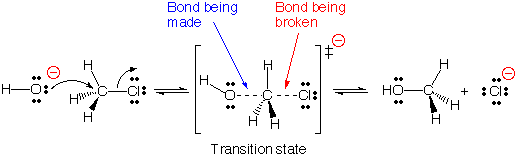 |
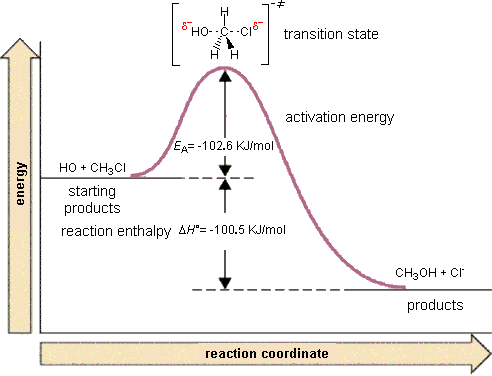
| Now consider the reaction of chloromethane with a hydroxide ion. The product is called methyl alcohol or methanol. An organic chemist would label this an SN2 reaction because it involves the substitution of one nucleophile (OH-) for another (Cl-) in a one step reaction. For the purposes of this discussion, we need to focus on the changes that take place during the reaction. As carbon accommodates 4 bonds (and almost always has 4 bonds) the attempt by hydroxide to form a new bond to carbon must be accompanied by the breaking of the carbon-chlorine bond. As illustrated, energy input is needed to break the bond and the reaction must go over a barrier (102.6 kJ/mol) while it is breaking the new bond. Since this is an exothermic reaction and as a result of the formation of the new bond, eventually the used energy is recovered and then some and 100.5 kJ/mol will evolve from the reaction. A temperature increase will increase the rate of reaction as more collisions will have sufficient energy to proceed over the barrier. Another possibility to increase the rate of reaction might be to add a catalyst which might lower the barrier. Note that with an activation energy just a little over 100 kJ/mol, this reaction will probably take place at a reasonable rate even at room temperature. |
 |
For those interested in more information about chemical reactions, please explore the examples that are given below for the classification system that includes: Decomposition, Combination, Combustion, Single Replacement, Double Replacement. These are the categories typically included in basic and general chemistry courses. It begins with a discussion of the writing of a chemical equation.
Balancing a reaction to make it an equation. A chemical reaction must contain the correct formulas of the reactants and products and mass must be conserved. If the nuclei are the same on both sides of the equal sign, then the mass is also conserved. In other words, if there are 4 oxygen atoms in the reactants, there must be 4 oxygen atoms in the products.
The first step in writing a balanced equation is to
write the correct formulas for reactants and products. Once this is done,
subscripts must not be changed to balance the equation as subscript or formula
changes change the
substance. Balance the equation using coefficients (numbers in front of the
formula). A coefficient denotes the relative number of moles of the substance
whose formula it precedes. Locate the formula with the largest subscript (not
subscripts within a polyatomic ion but subscripts that give the number of atoms
or ions per formula unit). For the reaction below, 8 is the largest subscript.
C3H8(g) + O2(g)
g CO2(g) + H2O(g)
Imagine the coefficient 1 in front of the propane
(C3H8) and balance the hydrogens and then the carbons.
1 C3H8(g) + O2(g)
g 3 CO2(g)
+ 4 H2O(g)
Observe that there are now ten oxygen atoms on the
right and that there are two oxygen atoms in an oxygen molecule on the left.
Divide the number of atoms needed (10) by the number per molecule or formula
unit (2) to arrive at the correct coefficient (5).
C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g)
Notice that coefficient of 1 is not written in the
final equation but is understood. Also notice the very common technique of
leaving the O2 (or H2) until last if it is present. The coefficient for O2
affects the amount of one element only whereas the other coefficients change the
amounts of at least two elements. For double replacement reactions, start with
the ion with the largest subscript. In case of a tie, choose the ion with the
largest oxidation number. For the reaction below,
BaCl2(aq) + Na3PO4(aq)
g Ba3(PO4)2(s) + NaCl(aq)
Na+ and Ba2+ have subscripts of 3 but Ba2+ has the
higher charge. Notice that the subscript 4 is part of the phosphate polyatomic
ion and is not part of this consideration. Start with the Ba2+ and work from one
side of the equation to the other. Balance the barium ions on the left side of
the equation by inserting a coefficient of 3.
3 BaCl2(aq) + Na3PO4(aq)
g
1 Ba3(PO4)2(s) + NaCl(aq)
The coefficient of 3 in front of BaCl2 locked in 6 chlorides so a 6 is now needed in front of NaCl on the right.
Reaction Types
Combination: the reaction of
two substances to form one substance.
MgO(s) + H2O(l) = Mg(OH)2(s)
SO3(g) + H2O(l) = H2SO4(l)
Decomposition: The reverse of combination or the
breaking down of one substance into two or more substances.
H2CO3(aq) = H2O(l) + CO2(g)
Ca(HCO3)2(aq) = CaCO3(s) + H2O(l) + CO2(g)
Mg(OH)2(s) = MgO(s) + H2O(g)
Combustion: The rapid reaction of a compound with
oxygen. Some combustion reactions are also combination reactions and vice versa.
2 CH4O(l) + 3 O2(g) = 2 CO2(g) + 4 H2O(g)
2 C8H18(l) + 25 O2(g) = 16 CO2(g) + 18 H2O(g)
Single Replacement: The replacement of an element in
a compound by another element originally in elemental form.
reaction, observation
Cu(s) + 2 AgNO3(aq) = Cu(NO3)2(aq) + 2 Ag(s) plating
of metallic silver on copper and appearance of blue color in solution
Mg(s) + H2SO4(aq) = MgSO4(aq) + H2(g) gas evolution
Double Replacement: An exchange of positive and
negative partners by two compounds (most commonly involving two ionic compounds)
in aqueous solution. These reactions usually proceed when at least one of the
products is a compound insoluble in water (precipitate), a gas or a compound
that decomposes into a gas, or has predominantly covalent bonds (unionized or
slightly ionized).
reaction, observation
3 BaCl2(aq) + 2 Na3PO4(aq) = Ba3(PO4)2(s) + 6 NaCl(aq)
white ppt.
K2CO3(aq) + 2 HNO3(aq) = 2 KNO3(aq) + H2O(l) + CO2(g)
gas
H2SO4(aq) + 2 KOH(aq) = K2SO4(aq) + 2 H2O(g) heat
An experiment that can be
performed in a well stocked kitchen but make sure you wear goggles.
Try this on a very small scale first before
increasing the amounts. Add a teaspoon quantity of baking soda to a large
glass or jar preferably in a sink. Add sufficient water and stir to
dissolve the baking soda. Add a small amount of vinegar to the baking soda
and observe. To make it even more interesting, add several drops of dish
washing liquid to the baking soda and water before adding the vinegar. The
baking soda is sodium bicarbonate (NaHCO3) and the vinegar is 5%
acetic acid (HC2H3O2)
and 95% water.
HC2H3O2
+ NaHCO3 = NaC2H3O2
+ H2CO3
H2CO3 = H2O
+ CO2
This is a two step reaction. The first
step is a double replacement reaction and the second is a decomposition of one
of the products, carbonic acid. You should notice fizzing as a result of
the production of carbon dioxide gas. When soap or detergent is also
present, enjoyable bubbles also are usually produced.
For some exercises on
classifications of reactions, please
visit
http://exercises.murov.info/ex7-1.htm
and do numbers 2,
4, 6, 8, 10, 14, 18, 22, 28.
QUANTITATIVE CONSIDERATIONS IN CHEMISTRY
The last section presented an introduction to a selected number of chemical reactions. Lavoisier was one of the first to do quantitative chemical experiments. He weighed the amounts of all the starting materials and products and found the total mass to remain unchanged and thereby proved that mass is conserved in chemical reactions to measureable limits. You might have noticed that the measureable limits qualification has been added when mass conservation is mentioned because there is a real goal of being as correct as possible. Because Einstein was amazingly able to equate mass and energy, it is now known that there are mass changes in chemical reactions. For exothermic reactions, a tiny amount of mass is lost and vice versa. However, a calculation of the mass changes come out on the order of one billionth of a gram and the smallest mass changes that can be detected are about a millionth of a gram. This not just a matter of building a better balance. Vibrations and other problems make the development of balances 1000 times more sensitive than those of today virtually impossible. Although it is difficult if not impossible to demonstrate that E = mc2 in chemical reactions, many other predictions made by Einstein's theories have been elegantly proven.
Chemical Calculations and the Mole. (This section is intended especially for those intending to take more chemistry courses.)
In a chemical equation such as N2 + 3 H2 = 2 NH3 , the symbolism tells us that 1 molecule of nitrogen (recall that nitrogen and hydrogen both exist in nature as diatomic molecules) reacts with 3 molecules of hydrogen to yield 2 molecules of ammonia. While enabling us to imagine the changes that take place during the reaction such as the breaking of nitrogen-nitrogen and hydrogen-hydrogen bonds and the formation of nitrogen-hydrogen bonds, on a laboratory scale, the reaction has limited value. It is not possible to run a reaction in the laboratory between one molecule of nitrogen and 3 molecules of hydrogen. The minimum our balances can weigh is about 10-5 g or 1017 molecules. Another way to look at the equation is that it gives you the ratio of molecules of nitrogen to hydrogen as 1:3. We could think of the reaction as stating that 1 dozen molecules of nitrogen reacts with 3 dozen molecules of hydrogen to give 2 dozen molecules of ammonia. These quantities are still trillions of times too small to be weighed. As a result chemists have defined a unit called a mole that make the amounts appropriate for weighing. Since the reaction gives the ratio, this would mean that 1 mole of nitrogen reacts with 3 moles of nitrogen to produce 2 moles of ammonia.
So if a dozen is defined as 12 units, how many units define a mole? The mole must be a large number so that a mole of a substance can be weighed on a laboratory balance. The number is called Avogadro's number and has at first glance, the weird value of 6.02214076x1023 or 602,221,407,600,000,000,000,000 units/mol or 602.22 sextillion units/mole. This number was originally determined by experimental determination of the number of carbon atoms in 12 grams of the carbon-12 isotope and hence the "weird" number but has recently been defined as the number of units in a mole. A mole of anything has this number of units just like a dozen of anything has 12 units. Looking back at the equation, 1 mole of nitrogen reacts with 3 moles of nitrogen to produce 2 moles of ammonia but our balances read out in mass or grams and not moles. To calculate the mass of 1 mole of nitrogen, the molecular mass of nitrogen is needed. The periodic table tells us that the atomic mass of a nitrogen atom is 14.007 amu. This means that a mole of nitrogen atoms would have a mass of 14.007 grams. Since there are 2 atoms of nitrogen per molecule of nitrogen, the molecular mass of nitrogen (N2) would be: (14.007 grams/mole of N)(2 moles of N)/1 molecule of N2) = 28.014 grams N2/molecule N2 . The use of units and ratios in this way is probably the most commonly used technique for carrying out chemical calculations. Ratios are set up so that units cancel and unit conversion factors inserted until the desired unit is attained.
As an example of a unit conversion, consider the example below that has the goal of calculating the number of cm in 1.80 meters. Notice that the goal is to use the unit conversion that will cancel the given units (m) and introduce the desired units (cm). When the unit conversion or ratio of desired units to given units is not immediately available, it is sometimes possible to string a series of unit conversions together to obtain the desired units. Notice in the following calculations that sequential diagonal cancellation of units using familiar unit conversions is utilized until the desired result is obtained. This technique has several different names including unit conversions, factor label, and dimensional analysis.
Unit Conversions: a very useful calculation tool.
A unit conversion is a ratio that represents equality or unity. Because of
the equality, any measurement can be multiplied by a unit conversion without
changing the value of the measurement. As a consequence of the change of
units, the numbers will also change but the value of the measurement stays the
same. As an example, suppose you are 1.80 meters tall and want to know
your height in centimeters. By definition, 100 cm = 1 meter.
Using algebra, we can divide both sides of the equation by 1 yielding 100
cm/1 m = 1 and 1 m/100 cm = 1. The ratio is 1 thus
the ratio is a unit conversion. We also could have divided both sides of
the equaion by 100 cm yielding the second unit conversion above Since unit
conversions equal unity, any unit conversion can be inverted and it will still
equal 1. Now we take the measurement of 1.80 meters and multiply it by the
appropriate unit conversion so that the given units will cancel and the answer
will have the desired units.
(1.80 m)(100 cm)/1m = 1.80x102 cm
This simple example could have been performed in your head but this procedure makes more complex calculations more straightforward. Suppose instead you wanted your height in inches. There are exactly (due to definition) 2.54 cm/1 inch and 1 inch/2.54 cm. It would be possible to multiply the 180 cm by 1 inch/2.54 cm or unit conversions can be strung one after another to convert the initial measurement to the desired units.
(1.80 cm)(100 cm/1 m)(1
inch/2.54cm) = 70.9 inches
For exercises in unit conversions, visit http://exercises.murov.info/ex1-2.htm and do numbers 1-11.
The amounts of reactants and products in chemical reactions are calculated using the same approach.
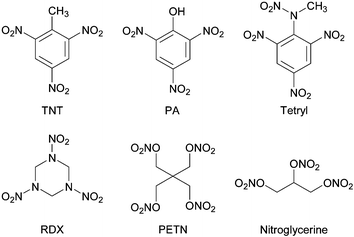
| The government of Germany realized as they prepared for WWI, that they would be confronted by a crucial shortage of some chemicals. In the figure to the right, what do these four common explosives have in common? Explosives are compounds that react very quickly, are highly exothermic and produce large quantities of gas. Conversion of a liquid or solid to gas involves a volume change of approximately 1000 times. The heat given off combined with a huge expansion of volume usually is the cause of the damage from explosions. Many but not all explosives including the those in the figure all contain a significant amount of nitrogen. The explosive reaction produces copious quantities of nitrogen and other gases. Remember that the nitrogen molecule has a triple bond and is unusually stable. Thus the reaction gives off a substantial amount of energy and a huge volume of gas. |
 |
As mentioned earlier, the earth's atmosphere contains 78% nitrogen. However, making nitrogen molecules into molecules useful for synthesis is extremely difficult because of the very high stability of nitrogen. Some plants are capable of fixing nitrogen but this has limited production capabilities. Germany like almost all countries had been importing nitrogen containing compounds from guano deposits rich in sodium nitrate from Chile but this source would end with the onset of war. Fritz Haber and Carl Bosch developed a process (1909) still used today for combining nitrogen and hydrogen to synthesize ammonia. N2 + 3 H2 = 2 NH3 . The reaction coordinate diagram to the right in a very oversimplified way shows how the conditions and catalysts used in the Haber process lowered the activation energy and enabled the large scale production of nitrogen compounds. Haber was a devout patriot and not only helped German produce explosives but is also considered a pioneer in the horrific use of chemical warfare making his positive contributions pale compared to his negative contributions. For more information about the Haber-Bosch process, visit: https://en.wikipedia.org/wiki/Haber_process |
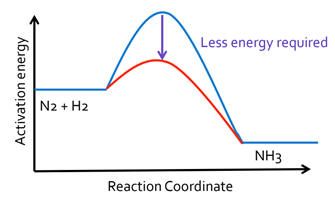 |
The subsequent steps to convert ammonia into nitrogen compounds
including explosives were already known so the Haber-Bosch process
enabled Germany to be competitive throughout most of WWI.
Lets now consider a hypothetical challenge faced by the
manager of an ammonia producing plant. Unlike nitrogen, hydrogen is not
available from the atmosphere and must be synthesized. Assume that the
plant manager has 56.0 grams of hydrogen gas and wants to know how much nitrogen
should be introduced into the reaction chamber. Because the balanced
equation gives the molecular or mole ratios but not the mass ratios, mass needs
to be converted first to moles followed by the use of the mole ratio from the
equation. Finally the number of moles of nitrogen needed is converted back
to mass.
(56.0 g H2) (1 mole H2/ 2.016 g H2)(1 mole N2/3 moles H2)(28.014 g N2)(1 mole N2) = 259 g N2
A similar calculation will give the expected amount of ammonia that should be formed assuming 56.0 go of hydrogen and 259 g of nitrogen are reacted:
(56.0 g H2) (1 mole H2/2.016 g H2)(2 mole NH3/3 moles H2)(17.03 g NH3)(1 mole N2) = 315 g NH3
It should be noted that the expected or theoretical yield of 315 g of NH3 might not be realized for many reasons. The reaction might also yield other products, it might not go completely from left to right and some of the product might experimentally be lost. For this reason, it is common to calculate the percentage yield of a reaction or the experimental yield divided by the theoretical yield x 100%.
One final example has been selected because it introduces another combination of units. Water is commonly used as a solvent in chemistry and the chemical of interest is often introduced by measuring an amount of the aqueous solution. Two common methods for expressing concentrations are molarity and percent by mass. A one molar solution means than 1 mole of the substance is diluted to (not added to) 1 Liter with water. For instance, a 0.10 molar sodium chloride solution could be prepared by adding 0.10 moles (5.84 g) of NaCl to a 1 L container and then diluted to 1 L with water.
Fluoride compounds are often included in toothpaste
formulations because of the ability of fluoride to protect teeth. Note
that fluoride like every chemical is toxic if too much is used and it is
important that the concentration be high enough to be effective but low enough
to avoid mottling of teeth and the toxic effects of fluoride.
Stannous fluoride [or tin(II) fluoride, SnF2] is one of the compounds used to
provide the fluoride. One of the methods of preparing stannous fluoride is
to react stannous oxide with hydrogen fluoride.
SnO
+ 2 HF = SnF2 +
H2O This double replacement reaction needs hydrogen
fluoride which is a gas at room temperature. The HF could be introduced as
a gas or somewhat more conveniently as an aqueous solution. Concentrated
hydrofluoric acid contains 49% HF by mass and has a concentration of 28.9 M (moles/L).
The formula mass of SnO is 118.1 g/mol + 15.999 g/mol =
134.7 g/mol. If 13.5 g of SnO are to be used in a reaction with HF, how
many milliliters of concentrated HF should be added to the SnO?
(13.5 g SnO)(1 mole SnO/134.7 g SnO)(2 moles HF/1 mole SnO)(1 L/28.9 moles HF)(1000mL/1 L) = 6.94 mL HF solution
For exercises on
formula mass, visit
http://exercises.murov.info/ex8-1.htm
and do
numbers 1, 7, 10, 17, 20.
For exercises on chemical reactions, visit
http://exercises.murov.info/ex8-3.htm and do
numbers 31, 33, 35, 36, 37, 39, 41.
It has been mentioned that in sodium chloride, the
sodium ion and chloride ions are strongly attracted to each other in a 3-D array
by ionic bonds. For bonds between metals and non-metals, most bonds are
predominantly ionic. However, for elements near the center of the periodic
table and especially for carbon and nitrogen, ionic bonds are uncommon at best.
Instead, these elements tend to share electrons and form covalent bonds.
In addition to carbon, hydrogen usually forms covalent bonds. Even oxygen
finds itself covalently bonded in most molecules and polyatomic ions. A
method that makes use of a scale called electronegativity is often used to
determine if a bond is ionic or covalent but this scale often gives incorrect
descriptions of bonding character. For the purposes of this coverage, it
will be assumed that bonds between a metal and non-metal or polyatomic ion is
ionic and bonds between non-metals are covalent with hydrogen considered to be a
non-metal. In addition, electrons between two non-metals will not be
shared equally resulting in polar covalent bonds unless both partners are the
same non-metal or the C-H bond. The extremely common C-H bond acts very
much like a non-polar covalent bond. This means that the H-O bonds in
water should be considered to be polar covalent.
For
some exercises on ions, please visit
http://exercises.murov.info/ex6-1.htm
and do numbers 7,8.
For
some exercises on bonding, please visit
http://exercises.murov.info/ex6-3.htm
and do numbers 1-14.
Water.
Water covers 71% of the earth's surface but only about 3% is fresh water. The value of water in life and chemistry cannot be understated. Water is commonly described as the "universal solvent" supposedly because it dissolves more substances than any other solvent. It is a very good solvent for ionic and small polar compounds but the claim that it dissolves more substances than any other solvent is disputable. The number of known chemical compounds is over 50 million but only 350,000 chemicals have been inventoried, only about 9000 are used in commerce and only about 900 have been tested for safety. Most known compounds are organic compounds and for all practical purposes, only those with 4 or fewer carbons have a chance at significant water solubility.
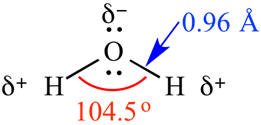
| Water has the
formula H2O
and because of its extreme importance, we need to know more about its
molecular structure. Since we should not make any assumptions, the
sequence of bonding could be H-H-O or H-O-H or a 3 membered ring. We
find in chemistry that symmetry is preferred and we also know that it is
extremely rare for hydrogen to form more than one bond. This leaves
H-O-H as the expected sequence and this has been experimentally
verified. Because oxygen has a greater affinity for electrons than
hydrogen, the bonds are not symmetric and the electron density is higher near
the oxygen resulting in polar bonds. Thus oxygen has a partial
negative charge and the hydrogens partial positive charges. Just as
important as the sequence is the geometry of the molecule. A
linear molecule would not have any net polarity and probably would not
be a good solvent. Experimentally water is found to be bent with a 104.5o
bond angle. The combination of charges and geometry results in a very
polar molecule that serves as a great solvent for ionic and small polar
molecules. FYI: 0.96 Angstrom units = 0.096 nanometers. |
  |
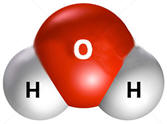  |
As a result of
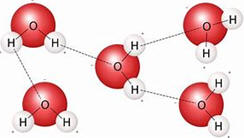
polarity, water has other unusual properties. The bottom right figure
above shows that the hydrogen from each water molecule because of their partial
charges are attracted to oxygens on adjacent molecules. This attraction is
a special type of intermolecular attraction call a hydrogen bond. Not only
are the properties of water highly dependent on hydrogen bonds, even your
genetic characteristics determined by the sequence of bases in DNA are
determined by hydrogen type bonds. For water, the hydrogen bonds mean that
water molecules are strongly attracted to each other resulting in a boiling
point that is over 100oC
higher than would be predicted based on similar compounds. Observation is the first and key step in the scientific method but most
of us need to improve our observational skills. How many times
have you observed a glass containing water and ice? Have you ever
been astounded by this observation and asked whey the ice is floating in
the water. As a liquid cools, the volume should contract and the
density increase and ice would be expected to sink in water.
However, the floating ice indicates that water behaves anomalously.
The hydrogen bonds in ice result in a 3-D geometry in
ice with the water molecules farther apart than they are in the liquid.
Water is one of an extremely small number of substances that expands
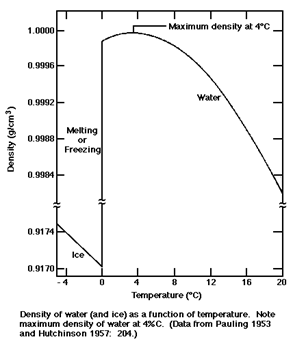
(experiences a density decrease ) when freezing. The graph to the right
demonstrates that the density of water does increase with cooling until
3.98oC is reached. At this temperature, water reaches its highest
density (at 1 atmosphere pressure) of exactly 1 g/mL = 1 g/cm3. We are fortunate that
this happens or life as we know it would probably be considerably different.
If water behaved normally upon freezing, lakes in the winter would still freeze
at the top because that is where it is coldest but the ice would then sink.
More ice would form on the top and sink and the lake would eventually end up
frozen solid making life in the lake difficult if not impossible to sustain.
Also note that the freezing of water results in a 9% drop in density.
This means that a full container of liquid water that is tightly capped
could break when frozen. Observation is the first and key step in the scientific method but most
of us need to improve our observational skills. How many times
have you observed a glass containing water and ice? Have you ever
been astounded by this observation and asked whey the ice is floating in
the water. As a liquid cools, the volume should contract and the
density increase and ice would be expected to sink in water.
However, the floating ice indicates that water behaves anomalously.
The hydrogen bonds in ice result in a 3-D geometry in
ice with the water molecules farther apart than they are in the liquid.
Water is one of an extremely small number of substances that expands
(experiences a density decrease ) when freezing. The graph to the right
demonstrates that the density of water does increase with cooling until
3.98oC is reached. At this temperature, water reaches its highest
density (at 1 atmosphere pressure) of exactly 1 g/mL = 1 g/cm3. We are fortunate that
this happens or life as we know it would probably be considerably different.
If water behaved normally upon freezing, lakes in the winter would still freeze
at the top because that is where it is coldest but the ice would then sink.
More ice would form on the top and sink and the lake would eventually end up
frozen solid making life in the lake difficult if not impossible to sustain.
Also note that the freezing of water results in a 9% drop in density.
This means that a full container of liquid water that is tightly capped
could break when frozen. The figure to the right shows 6 test tubes containing the liquid and solid states of 6 single substances. Only for the 3rd tube which contains water does the solid float. If you think about it, although there are millions of substances, water is the only one you have probably observed with liquid and solid present. Therefore, we accept the behavior of water as the norm when in fact its behavior is extremely unusual. |
  |
| THE SCIENTIFIC
METHOD In hindsight, it is often possible to analyze the procedure and steps that led to many scientific discoveries and advances. Scientists do not stop and ask what step they are currently on but generally the approach can be summarized by the steps listed below. The first step of the process, observation is the key to to the procedure but recognize that observation means more than just seeing or hearing but means that the observer asks questions about the observation such as is it consistent with expectations. The great scientists do not overlook the unexpected but strive to find explanations. 1. Observe and question. 2. Propose a hypothesis or explanation for the observation. 3. Devise experiments and perform tests that support or refute the explanation. Reproducibility of the observation and the tests is a must. 4. If the experiments support the explanation, the explanation becomes a verified explanation and is elevated to the level of theory. While the word theory is loosely applied by society to mean explanation, scientists reserve the word for explanations supported by testing. 5. A theory is always subject to further observation and testing. There is a need to be prepared to modify the theory or even develop a new explanation and theory if additional results are inconsistent with the theory. 6. Application of the theory. |
 |
ACIDS AND BASES, pH and SALTS
| Salts are ionic compounds that contain positive ions such as sodium (Na+) or calcium (Ca2+) and negative ions such chloride (Cl-) or polyatomic ions like nitrate NO3-). In the crystalline state, the positive and negative ions surround themselves in some kind of array as depicted earlier for sodium chloride. Many but not all ionic compounds have substantial solubility in water. When an ionic compound dissolves in water, the ionic bond cleaves and the positive and negative ions are each surrounded by a stabilizing sheath of water molecules. NaCl = Na+(aq) + Cl-(aq) CaCl2 = Ca2+(aq) + 2 Cl-(aq) |  |
When a strong acid such as hydrochloric acid (HCl)
dissolves in water, like ionic compounds, essentially complete dissociation ionization
occurs.
HCl = H+(aq)
+ Cl-(aq) . However, for weaker acids such as acetic
acid (HC2H3O2) or hydrofluoric acid (HF), the ionization is only partial (in these cases 1 to 10%) and many of
the acid molecules remain unionized in water HF = H+(aq)
+ F-(aq).
Many bases contain a metal ion that is ionically bonded to hydroxide ions
[e.g., NaOH, Ba(OH)2].
Compounds like these hydroxides that have significant water solubility form strong bases because
they increase the concentration of hydroxide in solution. Other compounds
like ammonia weakly ionize in water and form weakly basic solutions. NH3
+ H2O = NH4+(aq)
+ OH-(aq). Other ions "hide in disguise" and when
their compounds are dissolved in water basic solutions are formed (e.g., NaHCO3,
Na2CO3, Na3PO4).
Na2CO3
+ H2O = Na+
+ HCO3- + OH-
.The strength of an acid or base depends on the extent of its ionization and its
concentration.
Thoughts or pictures of a shark usually evoke fear. But
most sharks are not harmful to humans and shark attacks while dangerous and
sometimes fatal are extremely rare. People also often react negatively to the
word acid. Much of this reaction is due to a lack of thought or understanding as
we encounter acids frequently in our lives. In fact many of us start the day by
drinking orange juice, a mixture that contains citric acid. We also commonly put
vinegar and oil on our salads but vinegar contains about 5% acetic acid. Our
stomachs contain hydrochloric acid to facilitate digestion. While the word base
does not seem to invoke as much fear, strong bases like strong acids attack and
break down proteins and can be just as hazardous as acids. Acids and bases
require careful use and used incorrectly can indeed be very
corrosive. As part of our introduction to chemistry, we need to gain a better
understanding of acids and bases so that we can take advantage of their many valuable uses
and avoid hazards from misuse.
| There are several different definitions of acids used by chemists including ones named after Arrhenius, Brönsted and Lewis. For this discussion, the simplest and narrowest Arrheniuis definition that says an acid increases the hydrogen ion content of the solution will suffice. Strong acids such as hydrochloric and nitric acids (HCL, HNO3) dissociate or ionize into a hydrogen ion (H+) and a negative ion. Since a hydrogen atom is simply a proton and an electron (almost all hydrogen is the isotope with atomic mass of 1), a positively charged hydrogen ion is simply a proton. A proton is a very localized positive charge and will attract and surround itself with any negative charges in the vicinity. Most of our discussion will be confined to using water as the solvent. In water, the oxygens have a partial negative charge and orient themselves around the positively charged proton. In some books, the symbolism H+ is understood to represent a hydrated proton. In other books, the H+ is said to react with a water to form a hydronium ion [ H+ + H2O = H3O+ ]. Still other books combine the proton with several waters to make more complex ions. Here, H+ will be used to represent protons in water but it should be recognized that the proton is bonded to one or more waters. |  |
Using the Arrhenius definition, most acids are written with the
proton first such as hydrochloric acid, sulfuric acid and acetic acid: HCl,
H2SO4 and HC2H3O2 (C2H4O2). Notice that for acetic acid, hydrogen appears twice
in the first formula presented. The reason for this is to distinguish between
the two types of hydrogen present. The first hydrogen is bonded to an oxygen
with a polar covalent bond. This bond is capable of breaking when acetic acid is
dissolved in water as the proton is immediately stabilized by bonding to water.
The other three hydrogens are covalently bonded to carbons and do not ionize
under virtually any conditions.
As mentioned, acids are very important and useful but can be corrosive. The acid
in car batteries, sulfuric acid, is a strong acid and in concentrated form needs
to be handled with extreme care.
Concentrated or 18 M sulfuric acid is very dangerous and will quickly burn skin.
If even a small drop spills on a hand or arm, it needs to be washed off as
quickly as possible with a huge volume of water. Vinegar is usually about 5%
acetic acid which calculates to about 0.8 M. A 0.8 M solution of sulfuric acid
would still be corrosive with a hydrogen ion concentration about 1 M (above 0.8
M because sulfuric acid has two hydrogens per molecule) and must be handled with
care. Because acetic acid is a weak acid, the acid concentration is only about
0.004 M. At this concentration of hydrogen ion, the solution is not hazardous
and is used on salads. Because it is common for acidic solutions to have low
concentrations of hydrogen ion, an alternative scale for communicating acidity
has been developed. You have probably heard of the pH scale and perhaps even
used it to determine the pH of a pool or of soil.
|
While compounds described with the word acid in their names clearly increase the
hydrogen ion concentration when added to water, even water has some properties
characteristic of acids. Water slightly dissociates into hydrogen and hydroxide
ions. H2O = H+ + OH- ( or 2 H2O = H3O+ + OH- ) A pure water solution dissociates to the extent that the hydrogen and hydroxide concentrations are both 0.0000001 M (1x10-7M). To avoid the use of such a tiny concentration, the pH scale was developed. A pH of 7 represents an acid concentration of 1x10-7M. This is called a neutral solution and any aqueous solution with a hydrogen ion concentration above this is called at least slightly acidic and basic if the hydrogen ion concentration is lower than this value. In this context p is defined as -log10 but application of logarithms is beyond the scope of this coverage. Please refer to the figure to the right for a correspondence between acid concentration an pH. Because of the negative sign in the definition of p, any pH values lower than 7 represent acid solutions. Values of pH higher than 7 have hydrogen ion concentrations lower 1x10-7M and are called basic solutions as their hydroxide concentations are higher than 1x10-7M. Vinegar with a hydrogen ion concentration of about 0.004 M solution would have a pH of about 2.4. It is important to note that because of the logarithmic nature of pH, each change in pH by 1 unit is a 10 fold change in acidity. Also note that the very low concentration of hydrogen ion in pure water, 1x10-7M, means that tiny amounts of any hydrogen ion or hydroxide ion donors can significantly alter the pH. One drop of 10 M HCl (approximate concentration of pool acid) added to 1 L of neutral water, will lower the pH from 7 to 3.3. Also note that purified water unless freshly prepared will have a pH of about 6 as a small amount of carbon dioxide from the air dissolves in the water and forms carbonic acid. CO2 + H2O = H2CO3 = H+ + HCO3- (dissolved CO2 lowers pH) While the figure above indicates a pH range of 0 to 14 and some books indicate the range is 1 to 14, the actual range is limited by practical considerations. Since acids are limited to a maximum concentration of about 10 M, the practical lower limit of pH is about -1. The hydrogen ion concentration is linked to the concentration of hydroxide ion in aqueous solution. When the hydrogen ion concentration is high, the hydroxide is low and vice versa. |
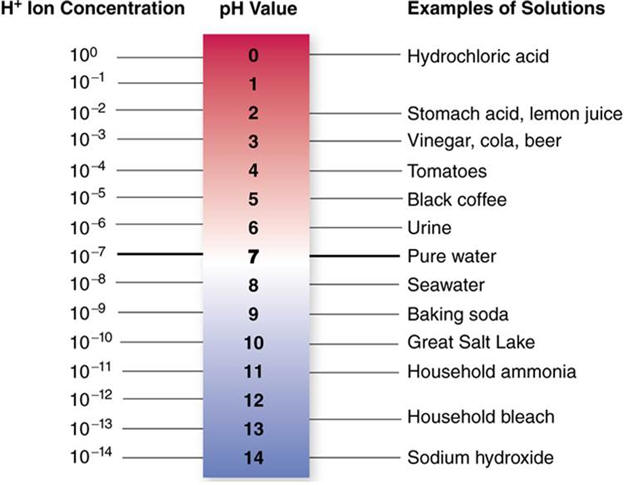 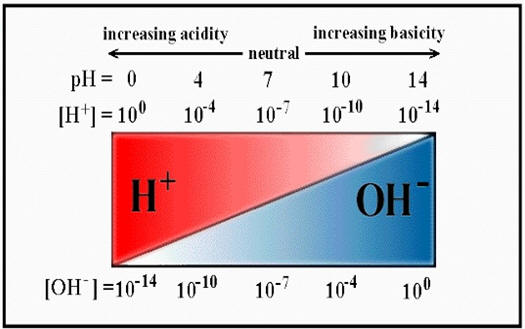 |
It is possible to combine an acid (or base) with a salt to form a solution of a
required pH that unlike the water example above when a drop of 10 M acid was
added, maintains a relatively constant pH when additional acids or base are
introduced. These pH change resistant solutions are called buffers and have
important roles in the laboratory and in biological systems. For example, your
blood is buffered to a pH in the range 7.2 to 7.4. When blood deviates from this
range, severe health consequences can result. When people drink orange juice
with a pH considerably out of the blood pH range, the buffers in the blood
prevent significant pH change making it safe to drink the orange juice.
Acid rain and other pollutants. The
burning of fossil fuels produces substantial amounts of carbon dioxide.
This issue will be discussed in a later section on climate change.
C8H18 + 25/2 O2 = 8 CO2 + 9 H2O
In an internal combustion engine such as the one in most cars, gasoline reacts
with oxygen to form carbon dioxide and water with the reaction illustrated for
octane above. Air contains 78% nitrogen and 21% oxygen and fortunately, the two
gases do not react at room temperature. Inside the engine cylinder, gasoline and
air are sparked to cause the explosion of the gasoline. The high temperatures
produced are sufficient to cause some nitrogen to react with oxygen to produce
nitrogen monoxide. N2 + O2 = 2 NO
2 NO + O2 = 2 NO2
The nitrogen monoxide is hopefully eliminated by the catalytic converter but
some makes it out the exhaust pipe and is further air oxidized to nitrogen
dioxide. Nitrogen dioxide is an orange, toxic gas that is one of the very
hazardous
constituents of smog along with ozone (O3). Los Angeles before the requirement of catalytic converters
used to be immersed in a cloud of orange smog that could easily be seen when
driving over a mountain pass into Southern California. In addition to the smog
problem, nitrogen dioxide combines with water in the atmosphere and contributes
to formation of damaging acid rain. Sulfur oxides formed during the
combustion of sulfur present in the coal also produce gases that contribute to
acid rain.
We observed when discussing chemical reactions that the study of reaction types is facilitated if classifications are developed in order to allow a focus on a smaller and similar number of reactions. Chemistry covers a huge range of topics and its study can be simplified by first categorizing chemistry into topics or processes. The traditional approach used in most colleges after the teaching a broad scope called general chemistry is to teach courses on organic, inorganic, analytical, physical chemistry and biochemistry. When classified by process, the classifications are often synthesis, analysis (identification and quantity), and physical chemistry. A short introduction like this cannot even try to cover all the branches but has as its goal, the introduction of some concepts that are encountered in our daily lives. In a typical general chemistry course, not much time is usually devoted to organic and nuclear chemistry but these fields will be briefly discussed because of their importance. Analytical chemistry and physical chemistry are essential for an understanding of chemistry but do not touch most of us quite as personally as organic and nuclear.
Until 1828 and even beyond, organic chemistry was considered
to be the chemistry of compounds derived from living things. It was
believed that chemicals derived from plants and animals contained a quality
termed vitalism that differentiated them from other compounds. In 1828,
Fredrich Wöhler was able to synthesize urea from substances that did not
originate in living things. He was able to demonstrate that the synthetic
urea was identical to urea derived from the living. Just like any
entrenched concept in any field, it took many years for scientists to throw out
the vitalism concept and accept Wöhler's research results. Eventually,
organic chemistry was redefined as the chemistry of compounds that contain
carbon. The vast majority of compounds that have been characterized do contain
carbon. Fredrich Wöhler deserves much of the credit for defining organic
chemistry and developing the concept of isomers . It is interesting to read
Wöhler's comments recorded in 1835:
Organic chemistry nowadays almost drives me mad. It gives me the impression
of a primeval tropical forest, organic full of the most remarkable things, a
monstrous and boundless thicket, a dreadful, endless jungle with no way of
escape, into which one may well dread to enter for there seems no way out.
During the last couple of centuries, great strides have been made towards
unraveling the mysteries of organic chemistry. While still “full of the most
remarkable things,” organic chemistry has been developed into a comprehensible
and fascinating science. Remember that 1835 was only 32 years after John Dalton
revived and considerably expanded on Democritus’ idea (ca 450 BC) that matter is
made up of atoms. It was truly a remarkable achievement that with the limited
concepts and techniques available, the early organic chemists were able to
discover many reactions and determine the structure of many compounds. Our
ability to study and probe atomic and molecular structures and chemical
reactions has progressed rapidly due to the development of techniques, such as
several types of spectroscopy. Dalton and Wöhler would be truly amazed by the current state of
organic chemistry. But don’t be misled! Despite the fact that research in
organic chemistry has enabled organic chemists to bring considerable order to
the study of organic chemistry, the field is and always will be a fertile area
for more research. Every discovery that is made enables the organic chemist to
seek answers to even more intriguing questions.
Carbon is at the center of most of the molecules in our body including sugars, amino acids, proteins, RNA and DNA. There are several important reasons for this. Carbon is in group 4A which means to acquire a stable inert gas structure of electrons, carbon would apparently have to lose or gain four electrons. This creates an ion with an uncommonly high charge of 4+ or 4-. Instead carbon shares its 4 electrons with up to 4 partner atoms and both atoms are generally more stable having 8 electrons in orbitals in the vicinity of their nuclei. This means that carbon almost always has 4 bonds. The bonds between carbon and the atoms of many elements and especially hydrogen, nitrogen and oxygen are often very strong. Perhaps even more important carbon forms strong bonds to other carbons. This can result in many structures including long carbon chains and even chains of hundreds or thousands of carbons. Within that chain, nitrogen and oxygen atoms are also often encountered. These possibilities mean that an unlimited number of compounds is possible. Adding to the complexity and beauty of organic compounds, the four bonds around carbon can be oriented in several different geometries making the number of possible compounds even higher. Creative science fiction writers are often seeking stories that will differentiate their writing from that of others. Since silicon is immediately beneath carbon in the periodic table, It is tempting to create a story with life on another planet based on silicon rather than carbon. This is a very unlikely possibility as silicon-silicon bonds are attacked by water and it is very difficult to imagine a living system that does not intimately involve water.
| To start this very brief introduction to organic chemistry, consider one of the simplest but very important molecules, methane (the major component of natural gas) or CH4. Since we have learned that hydrogen almost always forms only one bond, the four hydrogen atoms must be bonded to the central carbon. The initial inclination might be to arrange the hydrogen atoms symmetrically in a plane around the carbon as shown in the first figure. However, using the simple principle that electrons repel each other and try to orient as far as part as possible, the tetrahedral arrangement (far right) results and is confirmed by experiment. The bond angles are 109.5o instead of 90o. In general, when carbon is bonded to four different atoms, this 3-D arrangement depicts the position in space of the four atoms around the carbon, Often because of ease of presentation, methane is drawn like the planar figure. It is important to remember when you encounter this image that the actual geometry is a tetrahedral configuration. This also means that the structure for molecules like butane, all of the bonds about each carbon point towards the corner of a tetrahedron and that butane (see figure for next paragraph) is far from being a linear molecule. |
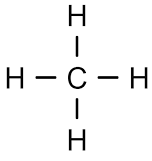  |
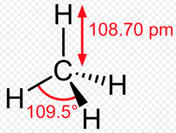  |
| As the carbon chain builds up, the first four compounds are: methane (CH4), ethane (C2H6 = H3C-CH3), propane (C3H8 = H3C-CH2-CH3) and butane (C4H10). However, for the formula C4H10 there are two options for the carbon skeleton. The carbons could be in a row or there could be a branch with the middle carbon attached to the other three carbons. Both of the butanes to the right have the same formula and molecular mass. Due to the different structures, the properties as shown below different. |  |
| compound | melting point (oC) | boiling point (oC) |
| n-butane | -138.3 | -0.5 |
| 2-methylpropane (isobutane) | -160 | -112 |
The case of the two butanes illustrates an extremely important concept called isomerism. When two or more compounds have the same formula but different structures, the compounds are called isomers. The two butanes differ in the sequence of bonding and are called structural isomers. We encountered this phenomena earlier when the isomerization of cis-but-2-ene to trans-but-2-ene was discussed. The 2-butenes have the same sequence of bonding but differ in geometry and cannot interconvert at room temperature. The 2-butenes are called geometric isomers or more generally stereoisomers. The existence of isomers considerably expands the number of possible compounds. Another very subtle but very important type of stereoisomerism goes unnoticed unless very careful observation and analysis is applied. The most common form of this isomerism occurs when a carbon is bonded to four different atoms or groups of atoms.
| Amino acids link together to form peptides and proteins that are vital for the existence of life as we know it. The generic formula for an amino acid is H2NCHRCOOH. Glycine is the simplest amino acid with a hydrogen for the R group and the formula H2NCH2COOH. For all other amino acids an R group that is not a hydrogen results in a carbon with four different groups and the existence of stereoisomers. The models to the right with the R group representing a methyl (-CH3) group show what at first glance appears to be two identical molecules. If you have access to models, make both models and rotate them in any direction, you will find that the molecules are not superimposable. |  |
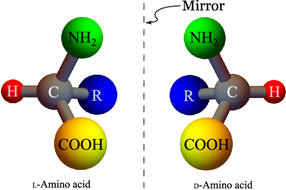 |
| Although the models are not superimposable, the figure to the right shows that the two molecules are mirror images. Molecules that are non-superimposable mirror images do have significant differences in their behavior. Besides rotating the plane of polarized light in equal but opposite directions, mirror images interact with other mirror images and receptor sites in different ways. As shown in the figure to the right, the two attacking molecules are mirror images. For the one on the left all three atoms approaching line up with the receptor sites. However for the one on the right, when the square is lined up the other two shapes do not. It is actually possible for the situation on the right to line up two of the sites but not all three. It is astonishing how important this concept of stereoisomers is in nature. | 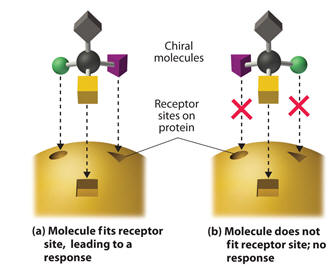 |
On earth almost all naturally occurring amino acids have the D configuration. If you were to eat food with only D amino acids, you would die of starvation because of your inability to metabolize L amino acids but not D amino acids. The D amino acids do not fit into the receptor sites in your enzymes. Sugars such as glucose have many stereoisomers and only those with the D configuration at carbon 6 are commonly found in nature.
| Sugars such as glucose have many stereoisomers
and only those with the D configuration at carbon 5 (carbon with yellow
-OH) are commonly found
in nature. Another set of 8 stereoisomers with the opposite
configuration at carbon 5 can be prepared but are very uncommon on earth.
Note that each intersection represents a carbon and four of the carbons
in each stereoisomer has 4 groups attached to it. Do not forget
that the configuration around each carbon is not planar as shown but
tetrahedral in shape. This means there should be 16 (2n
with n = 4) isomers and 8 are shown.
|
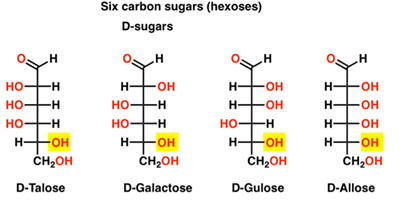 |
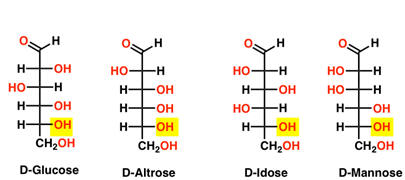 |
| Also, the head of the sugar grabs its tail to form 6 membered rings creating another carbon with 4 carbons. This is why there are two forms of glucose. | 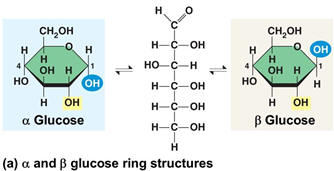 |
| There are many additional examples that point out the importance of stereoisomers. Many of you have probably smelled caraway seeds and spearmint. Did you know that the compound primarily responsible for the odor of both is a compound called carvone? Why do they have different odors then? The carvone in caraway is the mirror image of the the carvone in spearmint. The odor receptors in your nose are similar to the models above and one of the carvones fit into one type of receptor and the other into another receptor. As a result, your nose can distinguish between these two mirror images. | 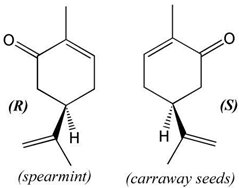 |
| Most of you are familiar with the drug ibuprofen that is the active component of many analgesic products. As seen in the figure to the right, ibuprofen has one stereogenic carbon and therefore has a pair of mirror images. The left one [S(+)] has anti-inflammatory properties but the right one does not. Fortunately in this case, the top one is not toxic and does not produce side effects and 60% of it is converted by an enzymatic reaction in the body to the active one. The lab synthesis produces a 50:50 mixture of the two. Because the separation is expensive and the presence of the inactive isomer does not cause problems, the product usually is sold as the racemic mixture. There is some evidence that the pure S(+) form is more effective than the racemic mixture. Therefore there are research efforts underway to find less expensive routes to the S(+) isomer. | 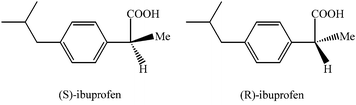 |
| Unfortunately, unlike the ibuprofen example, the enantiomer of the effective drug can have undesirable side effects. For these cases, it is necessary to run a synthesis that produces only the desired enantiomer. Another option is to separate the mirror images. Both of these options can be very difficult, time consuming and expensive. A very interesting and sad story involves the drug thalidomide. Do an Internet search for the sad history of this potentially useful drug. | 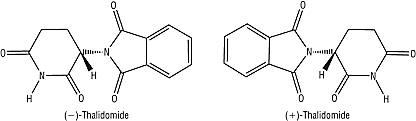 |
Finally on the intriguing topic of stereoisomers, it is interesting to question why life on earth has evolved with essentially one set each of amino acids and sugars. While there have been some explanations advanced, none can yet be advanced to the level of a theory.
| Consider the water molecule with the formula, HOH. If a hydrogen in the water is replaced with a methyl group, -CH3 the resulting organic compound is called methanol or methyl alcohol. Methanol or wood alcohol is unfortunately often present in moonshine and it can cause blindness. If instead, the hydrogen is replaced with an ethyl group, CH3CH2- , ethanol or ethyl alcohol results. While oxidation of methanol yields toxic formaldehyde and formic acid, oxidation of ethanol yields innocuous acetic acid (5% of vinegar). Ethanol is the compound in alcoholic beverages that causes significant controversy. Ethanol impairs driving skills and leads to the deaths of thousands of Americans annually in auto accidents. The U.S. prohibited its use from 1920 to 1933 but this well intended effort generally resulted in more harm (including criminal activity) than good. Adding another carbon results in two structural isomers, n-propanol and isopropanol. The latter makes up about half of rubbing alcohol. | 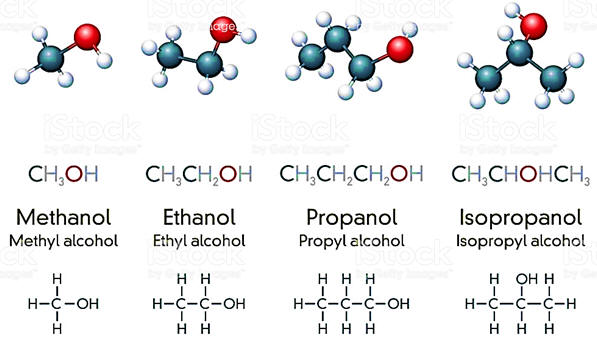 |
| Doctors and researchers have been searching for the ideal painkiller forever. Aspirin has some good properties but thins the blood and can cause Reyes in children. Morphine and its derivatives are much stronger than over the counter analgesics but have some alarming side effects including the terrible probability of addiction with overuse. Pharmacologists have attempted to modify the structure in a way that maintains the pain killing properties but without the addiction issues. Many prescription drugs available are the minimally successful results of these efforts. Even the use of too commonly prescribed vicodin (hydrocodone + acetaminophen), demerol, and oxycodone can lead to addiction. Note the similarities of the structures but it appears the pain relieving site must also be intimately connected to the site that causes addiction. Since this appears to be the case, it will probably be necessary to find a different basic structure for a non-addictive pain killer. |
 |
 |
Polymers
In the 1967 classic movie, The Graduate, the following conversation was heard
between the main character, Benjamin Braddock (portrayed by Dustin Hoffman, a 29
year-old playing a 21 year-old in his film debut), the guests and a family
friend, Mr. McGuire, at Ben’s college graduation party:
Guests: We're all so proud of you, proud, proud, proud, proud, proud, proud,
proud. What are you going to do now?
Ben: I was going to go upstairs for a minute.
Guests: I meant with your future, your life.
Ben: Well, that's a little hard to say.
Mr. McGuire: I just want to say one word to you - just one word.
Ben: Yes sir.
Mr. McGuire: Are you listening?
Ben: Yes I am.
Mr. McGuire: 'Plastics.'
Ben: Exactly how do you mean?
Mr. McGuire: There's a great future in plastics. Think about it. Will you think
about it?
Ben: Yes I will.
Mr. McGuire: Shh! Enough said. That's a deal.
Mr. McGuire correctly predicted and, if anything, understated the impact
plastics would have on our lives. The synthesis of improved plastics engages a
significant percentage of all research chemists. Many additional chemists work
on the characterization of natural polymers such as DNA, RNA and proteins. From
the paper of this book, the plastic of your pen and computer, the fibers of your
clothes to your hair and skin, polymers make up a significant amount of the
structural material surrounding us. Find a bunch of paper clips and connect them
together to make a chain. You have just constructed a model of a polymer. The
linking of molecules (or monomers) with chemical bonds end to end hundreds of
times results in the formation of a polymer. The structural material of plants,
cellulose, is a polymer of the sugar, glucose. The structural material of
animals, protein, is a polymer made from amino acids.
 |
polystyrene |
| Polyesters and nylon belong to another class called condensation
polymers. Nylon was discovered in 1934 at Dupont by Wallace Hume
Carothers (1896-1937). Nylon 66 can be made in the laboratory by
reacting adipoyl chloride with 1,6-diaminohexane. Notice that after one
molecule of each reacts, the two ends are still reactive and the left
end will react with another 1,6-diaminohexane while the right reacts
with adipoyl chloride. The process can continue hundreds of times. Your
instructor should demonstrate this reaction. For more information on
nylon, please visit: https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/carotherspolymers.html https://www.sciencehistory.org/historical-profile/wallace-hume-carothers 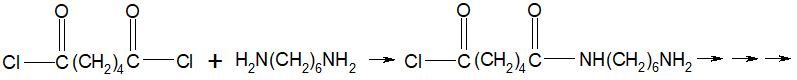 |
nylon 66 |
 |
For exercises on isomers, visit http://exercises.murov.info/ex16-1.htm and do numbers 5-7.
A basic understanding of nuclear chemistry is important for several reasons. Nuclear weapon capabilities significantly threatens the stability of all countries and limits our ability to achieve peace of mind. North Korea already has joined the several countries with nuclear weapon stockpiles and also has some delivery systems close to completion. Iran was working to produce sufficient bomb grade materials although this effort was slowed by a nuclear arms treaty. With the withdrawal from the treaty by the U.S., it is not clear if the treaty is still be followed by Iran. Additionally, the earth faces the challenge of global climate change. Some think that nuclear energy facilities could help to decrease the threat of fossil fuel caused climate change. To understand these issues, we need to expand upon a very brief discussion of isotopes that was presented early in this discussion.
I
| Isotopes are atoms of the same element with different numbers of
neutrons. Neutrons play a vey minor role in determining the
physical and chemical properties of an element but are crucial in
determining the nuclear stability of the element. Only very
limited proton to neutron ratios result in stable nuclei as illustrated
by the black dots in the chart to the right. If a proton to
neutron ratio is not a stable one, the nucleus will be radioactive and
over some time period will undergo a process that changes the proton to
neutron ratio. Ranges of half lives are given by the color of the
unstable isotopes. For the light blue region, the isotope is too
unstable to be produced. The most common radioactive decays are
alpha and beta decay. Neither of these decays necessarily leads to
a stable nucleus and often a sequence of several decays are required to
reach a stable nucleus. Radioactivity can be dangerous as high
energy particles and radiation are part of the process. These
emissions cause damage to living tissue and one of the results can be
cancer. A significant dose is potentially lethal. For elements with 43, 61 and 83 or more neutrons, no stable isotopes have been discovered. Some such as bismuth have such long life times, that radioactivity is not a danger. One of the isotopes of technetium (#43) has ideal properties as a medical tracer and is commonly used for diagnostic purposes. Many of the isotopes on the high end are very difficult to prepare and typically live only mico-seconds. |
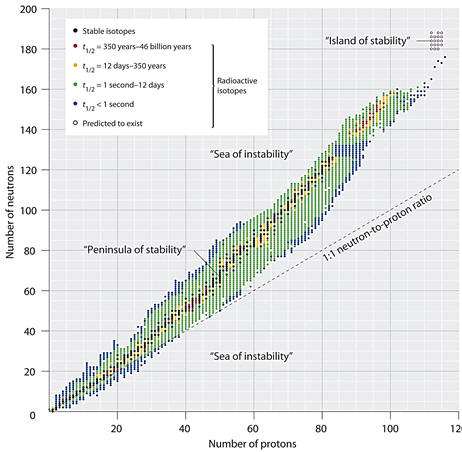 |
Examples of several stable and radioactive nuclei are given below along with the pathway of radioactive decay.
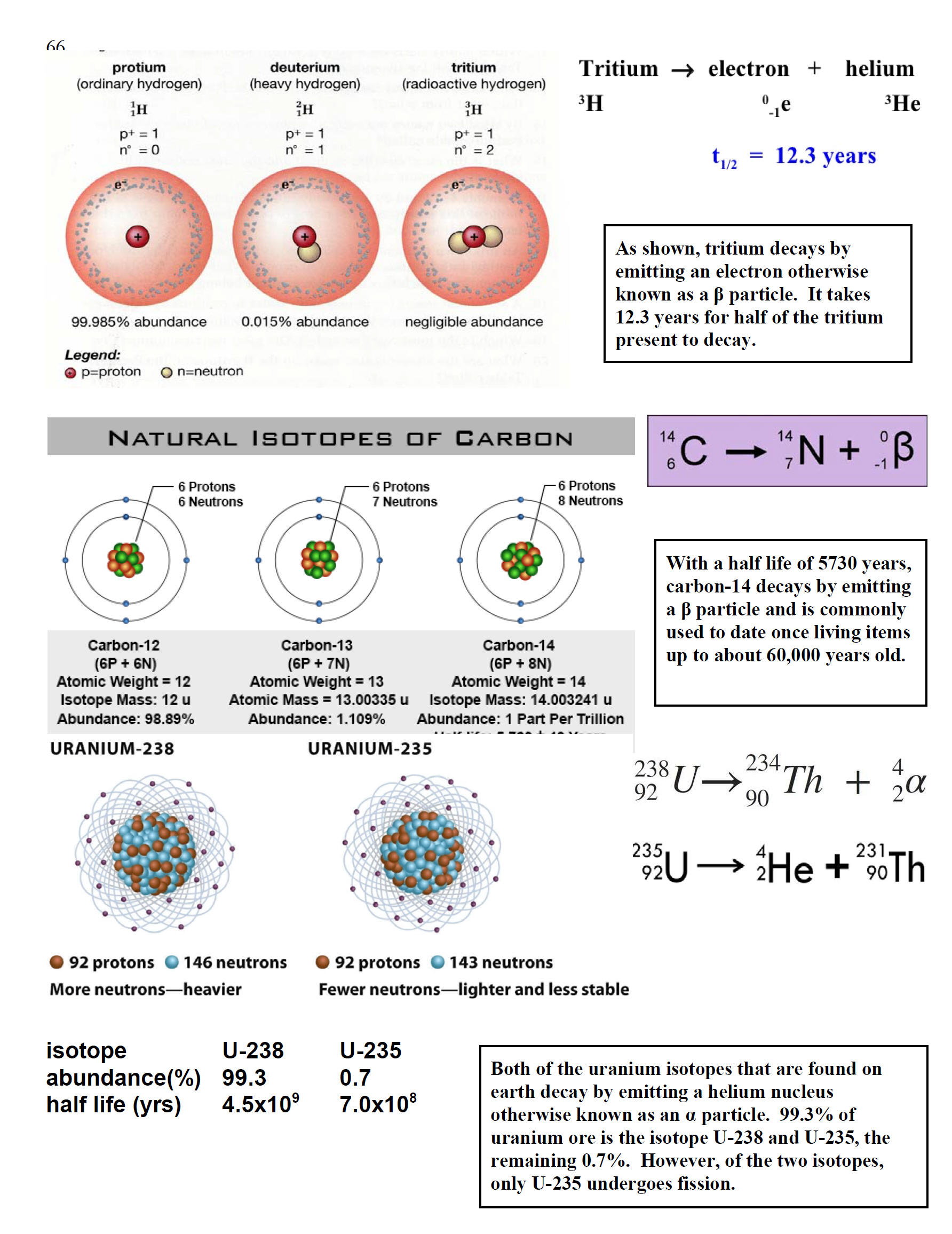
| Notice that there are predominantly two isotopes of uranium. The periodic table includes an atomic mass of 238.03 g/mole (or amu/atom) for uranium. This strongly indicates that most uranium on earth is U-238. Experimentally, naturally occurring uranium is composed of 99.3% U-238 and 0.7% U-235. In the late 1930's, it was discovered that when U-235 is impacted by a neutron, the uranium nucleus cleaves by one of at least two pathways and yields the nuclei of two smaller elements along with 2 or 3 neutrons. If these neutrons go on to impact more fissionable nuclei, more neutrons will be released. One neutron can initiate the reaction but the release of more than one neutron by the reaction means that the reaction can continue in an escalating way or a chain reaction that leads to a nuclear explosion. To sustain the chain reaction, a critical mass must be present or neutrons will go un-captured and the reaction will not be sustained. If the numbers of neutrons released is controlled, it is possible to use this reaction as a source of energy that can be converted into electricity. The products weigh slightly less than the reactants resulting in the release of substantial energy. Unlike chemical reactions where the mass change is too small to measure, the mass lost here is much greater and the amount of energy released is much more than for a chemical reaction. |
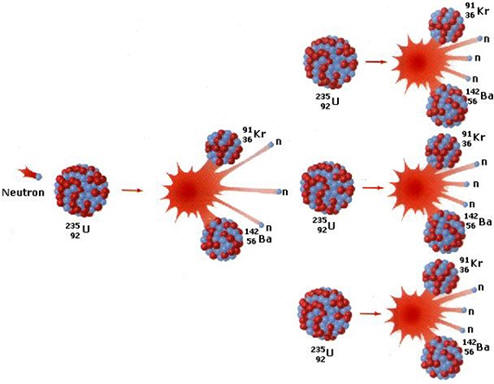 |
| Because U-238 does not undergo fission, to
build a nuclear reactor or a bomb, it is necessary to start with uranium
ore that contains only 0.7% U-235 and enrich the U-235 to a sufficient
percentage that a nuclear reaction can be sustained. Because the
two uranium isotopes have very similar physical and chemical properties,
separating the isotopes is a technologically difficult, expensive and
time consuming process. Put very simply, one of the techniques
involves making the uranium into UF6 and taking
advantage of the property that it can be converted into a gas at a
reasonably low temperature. The slightly lighter gas molecules of UF6
with U-235 move a little faster than those of U-238. By repeating
a procedure that takes advantage of this velocity difference many times,
it is possible to enrich the uranium in U-235 enough for a nuclear
reactor and more for a bomb.
The controversial Iran nuclear treaty involves the issues discussed above. Iran
would like to enrich uranium and they have the centrifuges needed to do this.
Iran claims that they want to enrich the uranium enough to use in peaceful
nuclear energy facilities. Bomb enrichment requires even higher percentages of
U-235 than energy facilities require. The treaty restricts Iran to preparing the
U-235 sufficient for energy facilities but insufficient for bomb use. Some
people and countries do not believe that Iran will abide by the rules. Unfortunately, nuclear fission is fraught with many problems. Radioactive waste is created that is dangerous for thousands of years and disposal and storage methods have not been satisfactorily addressed. There have been three major nuclear accidents at nuclear plants although new designs should reduce the risk. While it might appear that fuel is available, the enrichment of U-235 or the production of Pu-239 both have significant cost. There is also the real problem that weapons grade fissionable material could get into the wrong hands and result in a disastrous situation. |
| Although U-238 does not fission when impacted by neutrons, neutrons are captured which convert the U-238 into Pu-239. Pu-239 does undergo fission and can also be used to make bombs. At the end of WWII, two bombs were dropped in Japan. One was a U-235 bomb and the other was a plutonium bomb. Other isotopes of heavy elements also undergo fission. |
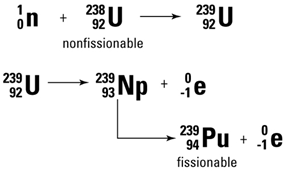 |
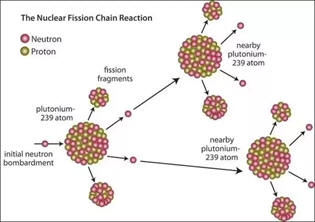 |
| It was mentioned above that the fission process is accompanied by a mass loss that results in a huge energy release. The graph to the right shows the stability of nuclei as a function of atomic mass. Iron-56 is the most stable nucleus. When uranium undergoes fission, two lighter and more stable nuclei are produced along with the release of energy. Notice that there is a much greater move toward stability when hydrogen atoms are fused and produce nuclei such as helium that are more stable than hydrogen. This is the process illustrated on the far right that occurs in the sun. The process shows the production of He-4. Recall that 91% of the atoms in the universe are hydrogen atoms and most of the rest is helium. Additional fusion reactions in stars produce elements up through iron. Several countries have been able to design and construct bombs based on nuclear fusion reactions although not with the sun fusion reaction. Considerable research continues to find ways to harness nuclear fusion. If and when successful, nuclear fusion could be an answer to our energy and climate change issues. Fusion has minimal nuclear waste issues, cannot undergo a meltdown type of event and fuel is cheap and abundant. | 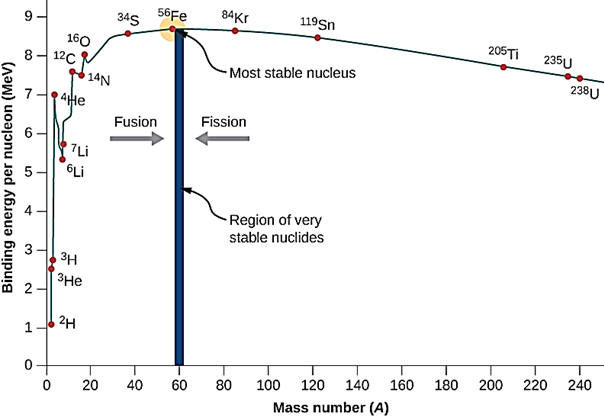 |
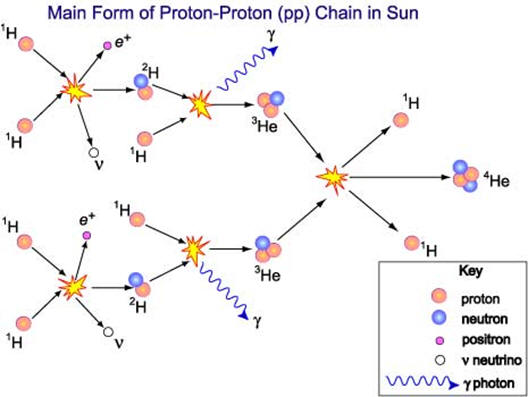 |
For exercises on
nuclear chemistry, visit
http://exercises.murov.info/ex15-1.htm
and do
numbers 1-4, 12, 13.
Arguably the biggest challenge currently confronting society is the high probability that fossil fuel combustion is causing an increase in average global temperatures. In addition to the hazards of extreme heat, increasing temperatures cause glaciers to melt, sea levels to rise, more damaging weather related events including droughts, wild fires, severe tornados and hurricanes and more.
| Before continuing on the climate change issue, it will be worth the time to consider the ozone depletion problem as a model. There are three formulas of oxygen that can exist - O, O2 and O3 (ozone). O and O3 are very reactive and toxic. Diatomic oxygen composes about 21% of our atmosphere and It is difficult to imagine a type of life that could exist without O2. Ozone is produced as a result of combustion processes and is a primary and dangerous constituent of smog. However, O3 plays a very important role in the upper atmosphere by filtering out substantial amounts of harmful UV radiation. In the 1970's, chemists provided evidence that freons used as spray propellants and in air conditioning units were causing a depletion of the very vital ozone layer. Fortunately, global governmental action has substantially decreased the use of the harmful freons and the ozone layer is slowly recovering from its hazardous decrease (lower ozone results in more skin cancer). Many people connect the ozone depletion issue with climate change. However, the issues are not related except that freons do cause both. The global community needs to understand the importance of the precedent set by the global response to the ozone issue and take similar action to remedy the even more serious climate change issue. | 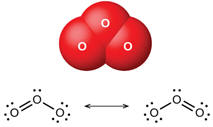 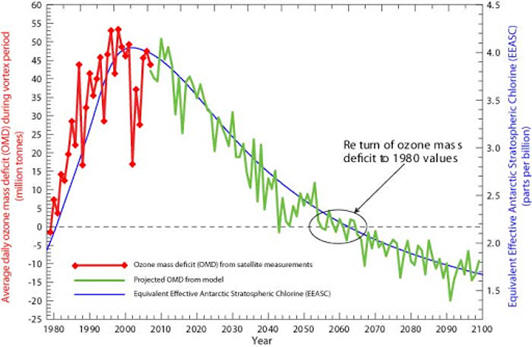 |
| Carbon Dioxide. The graph to the right clearly and definitively shows that the carbon dioxide (CO2) content (blue line)of our atmosphere has increased from a value of 280 ppm (or 0.028 %) in preindustrial time to about 387 ppm (or 0.0387% and rising) in 1991. Currently the value is 415 ppm or a 48% increase in CO2 concentration in the atmosphere since the industrial revolution began. The strong correlation between the CO2 increase and temperature increase (red line) demonstrates the strong connection between global temperature and atmospheric CO2 concentration. The correlation does not answer the question of which is the cause and which is the effect. However, notice also in this graph the additional good correlation of the temperature and CO2 curves with the amount of CO2 emissions. This provides very strong evidence that fossil fuel combustion is responsible for both the increase in CO2 in the atmosphere and the resulting global temperature increase and all of the accompanying negative consequences. The observed increase in temperature cannot be explained using natural astronomical variations but is completely consistent with scientific modeling that takes into account human contributions to atmospheric changes. Observed sea level increases, ice mass loss and changes of animal habitats add further evidence that Greenhouse gases are dangerously impacting our environment. |
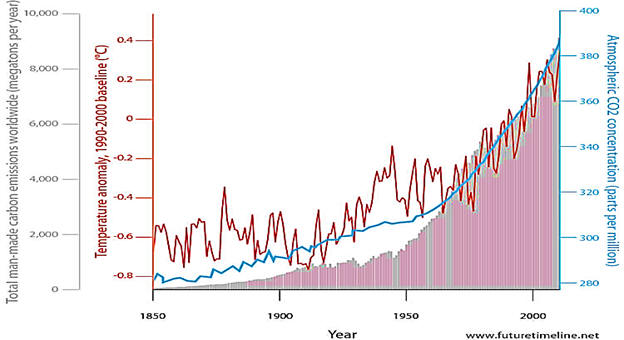 |
| Name the four most abundant gases in
dry air (the humidity varies widely by location and time of the year
from a trace to 4%) in decreasing order of abundance. Many people do not name nitrogen as the most abundant gas, extremely few name argon as the third most abundant gas and CO2 is commonly named as one of the three most abundant gases. Presumably, the latter is a conclusion reached because plants need CO2 and animals exhale CO2. However, only 3% of human exhaled gas is CO2 and most is N2. Why? |
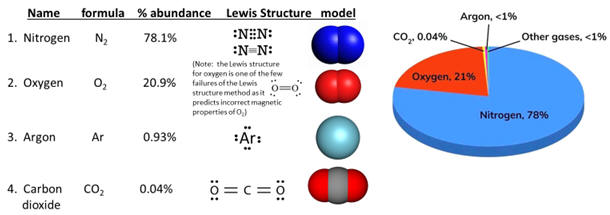 |
Global Warming. What are the environmental changes that result from changes in atmospheric composition? Both CO2 and CH4 absorb infrared radiation emitted from the earth. For some of the science supporting this, please refer to the addendum. Increases in the concentrations of these gases results in the trapping of more energy and higher temperatures on the surface of the earth. What are the consequences of global warming? Warmer temperatures are already severely affecting the health of hundreds of thousands if not millions of people. Deaths from heat waves are on the rise. The allergy season has already been extended an estimated 3 weeks/yr. Agriculture is affected and rain patterns are changing with very damaging drought a common problem. Animals are being forced to change their habitat locations. Wildfires and freaky weather have become much more common and both are wreaking havoc and mayhem around the globe. |
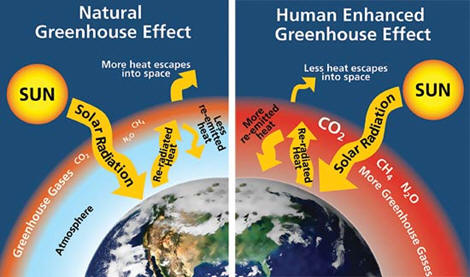 |
Habitats of several bird populations have changed by hundreds of
miles as a result of global climate change. |
 |
| SEA LEVEL RISE. Increasing atmospheric temperatures cause the temperature of the oceans to rise and the glaciers and ice masses of the earth to melt. An increase in ocean temperature causes a decrease in the density of water and an expansion of volume. Ice melting also causes an increase of ocean volume. Combined, the increase of volume is a severe threat to all coastal communities as shown in the figures below. The maps below show the devastating effects of 20 feet of sea level rise in a few locations on the earth. | 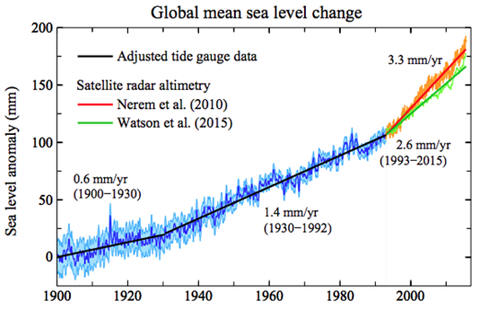 |
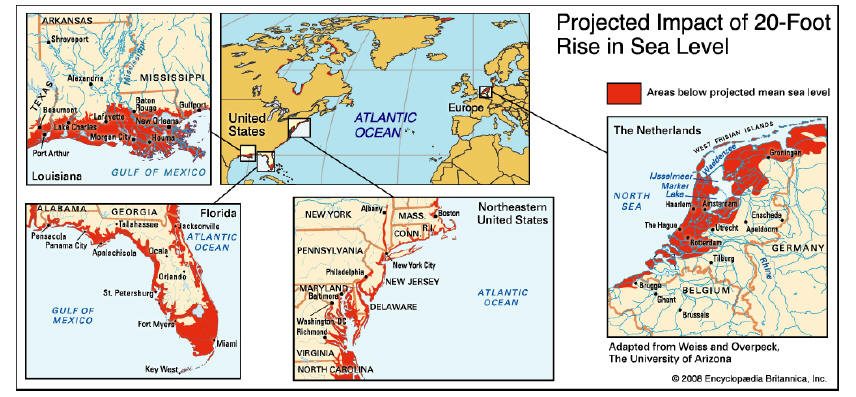
| ICE MASS LOSS. The figure to the right show
that the ice masses of the earth are undergoing a rapid melting that poses many
more threats to life on earth. In addition to raising the sea level and
threatening coastal communities, the arctic ice mass loss threatens to eliminate the
habitats of polar bears and walruses. Many populations of people will lose their
source of fresh water if the glaciers disappear. On the far right, the
long sought after Northwest Passage can now be navigated by cruise
ships due to the shrinking of the polar ice cap in August of each
year. |
 |
 |
| Ocean Acidification The atmospheric CO2 increase would be much bigger were it not for CO2 absorption into the ocean. CO2 is absorbed by the oceans and converted into carbonic acid. This results in an increase in the acidity of the ocean and a decrease in the pH. Already, the ocean pH has dropped from about 8.2 to 8.07. A pH decrease is a direct threat to all shell fish and coral reefs. Observations indicate that the Australia’s Great Barrier Reef is disappearing and will be gone in a few decades. |
 |
| The global temperature map to the right https://data.giss.nasa.gov/gistemp/maps/index_v4.html summarizes our current dilemma. With the exception of the continent of Antarctica, all of the continents are experiencing warming. The reddish regions are already 3oC warmer than 100 years ago. If this trend is allowed to continue, many species of life will go extinct and extreme suffering with substantial loss of life will result for us. Do we want the earth to resemble Venus, a planet with a runaway Greenhouse effect? We must change our behavior and confront the challenge. SOLUTIONS. Steps must be taken to phase out the use of fossil fuels rapidly. 1. One step towards achieving this goal is to initiate a carbon neutral fee and dividend on the use of carbon based energy sources. 2. Strongly encourage conservation. 3. Strongly encourage development and use of more solar, wind and tidal energy sources. Require that new construction include solar panels for at least 80% of their energy use. 4. Strongly encourage the use of hybrid vehicles and electric vehicles when electricity is not generated using fossil fuels. 5. Invest significantly in energy research to improve solar panel efficiency and to develop new energy sources such as nuclear fusion and artificial photosynthesis. |
 |
Virtual textbook (e-books) -
http://murov.info/chemessentials.htm
http://chem1.com/acad/webtext/virtualtextbook.html
http://cnx.org/contents/L1jDf6kt@5.6:BF5q2xlS@7/Preface-to-Concept-Development
http://mypchem.com
https://chem.libretexts.org/Bookshelves
https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)
https://chem.libretexts.org/Bookshelves/Introductory_Chemistry
https://chem.libretexts.org/Bookshelves/General_Chemistry
https://www.youtube.com/results?search_query=chemistry
https://en.wikibooks.org/wiki/General_Chemistry
http://virtuallaboratory.colorado.edu/CLUE-Chemistry/overview.html
also see: http://pubs.acs.org/doi/abs/10.1021/ed300456y
https://www.oercommons.org/search?f.search=chemistry
https://cnx.org/contents/havxkyvS@12.2:uXg0kUa-@5/Introduction
https://openstax.org/details/books/chemistry-2e
https://opentextbc.ca/chemistry/
https://www.khanacademy.org/science/chemistry
The exercises referred to above are among the many exercises available at: http://exercises.murov.info/chemexercises.htm .

hit counters
started 5/26/20, last modification 9/13/20
unique visitors