


Searching for Chemicals: A Material Science Lesson for Chemistry Students. Part 2
This Exercise is #4 in Experiments and
Exercises in Chemistry Courses
http://murov.info/chemexpts.htm
For Part 1 and 3, please jump to http://murov.info/matsci1.htm,
http://murov.info/matsci3.htm
For more web sites by Steven Murov including chemistry sites, jump to:
http://murov.info
Note 1
This site focuses on the exciting field of material science from a chemistry perspective. The thought processes involved in these exercises should serve to stimulate interest in chemistry and fill a sometimes overlooked part of the challenges in the field of chemistry. The first part of this lesson provided experience with some of the Internet resources available. It introduced and reviewed some of the sources of information available at the fingertips of anyone with an Internet connected computer. The exercises in the first website are more traditional in nature whereas the challenges in this site are more unique and hopefully will stimulate interest and insight into the selection of materials for specific applications.
Searches for materials based on desired properties.
A. Extreme Properties of Elements.
B. Material Selection Using the Internet: Search techniques
E. Compounds (evaluation of commercial products)
Appendix A. The Properties of Substances (Elements, Compounds)
Appendix B. The Properties of Solvents
Appendix C. Search for Compounds from Properties
Appendix D. Spectra of Substances
Appendix E. Links to Information about Metal Alloys
Appendix F. Solutions to problems
A. Extreme Properties of Elements. Generally speaking, when searching for a substance with desired properties, the search is not be limited to elements. However, for the purpose of the first part of this exercise, use of the website http://environmentalchemistry.com/yogi/periodic/ or https://periodictable.com/Properties/A/CrustAbundance.html (click on desired property) does make it straightforward and easy to determine if there are any elements that satisfy the property requirements. Use one of the web sites to determine which elements have the properties listed in the top row of the table.
| density>20 g/cm3 | melting point >3000 K | boiling point 0 to 90 K | electrical conductivity 0.37x106 to 1x107cm-1 W-1 |
B. Material Selection Using the Internet: The search techniques. The very interesting and increasingly
important field of material science involves determination of the properties of
a material needed for an application and then selection of a material that has
the desired properties. A
very useful site for selecting polymers and metals for particular applications
is:
http://www.matweb.com/ or
http://www.matweb.com/search/PropertySearch.aspx.
Other sites that are useful for finding the properties of the elements are:
http://ww.knowledgedoor.com/
https://environmentalchemistry.com/yogi/periodic/.
Before searching for a material, you should familiarize yourself with the
matweb site,
its capabilities and some of the properties in its database. To begin this
process, the exercises below will help you learn how to navigate around the
site.
1. Comparison of aluminum, iron and titanium. Determination of some properties of the elements. At the matweb site, enter the name of the element. Then click on the top entry to find the desired properties. For prices, try: https://en.wikipedia.org/wiki/Prices_of_chemical_elements
| Element | density (g/cm3)) | m.p. (oC) | hardness (Vickers) | Modulus of elasticity (GPa) | electrical resistivity (x10/strong>6 ohm cm) | cost ($/kg) |
| aluminum | ||||||
| iron | ||||||
| titanium |
(For filled in table, go to: III-B-1)
a. Until 1886 aluminum was an expensive metal that was not commonly used. What properties of aluminum often make aluminum the metal of choice for many applications? What changed around that time that made aluminum much less expensive and common for many uses? (III-B-1-a for answer)
b. According to the data you have entered in the table, titanium has some advantages over iron and aluminum. What are the pros and cons of titanium use and why is it not used more often? (III-B-1-b for answer)
| water absorption (%) | Vical softening point (oC) | visible transmission (%) |
3. Now lets do a search for a type of plexiglas that is needed to make a container for a visible transmission study at relatively high temperature. A search is possible for three different properties at: http://www.matweb.com/search/PropertySearch.aspx . For instance, you might select a minimum Vical softening point temperature of 115oC, visible transmission minimum of 92% and 0.4% water absorbance. 1705 choices will result. The criteria could be narrowed but another method to proceed is to register (free) on the site and select Advanced search. This gives you the option of entering plexiglass in the search text. Then enter two more criteria such as Vical softening at a minimum of 115oC and 0.4% water absorption. An even more selective option is to purchase the premium version which gives 10 criteria to further limit possibilities.
a. List the two results of the last search. (Answer at: III-B-3-a)
4.
Material Selection. Consider for a moment that you have been asked to select
a material to use as electrical wire. Assume for the this sample exercise that you are
restricted to the use of a pure element. First, you need to think about the
pertinent properties involved in this selection. For electrical wiring,
the element should: have very high electrical conductivity (or minimal
resistivity), be very ductile so it can be drawn into wires, have corrosion
resistance and have a low cost. Next, the relative importance of each property
needs to be considered and then a search should be performed for elements that
satisfy the criteria. Taking all properties into account, you would probably
conclude that copper and aluminum would be the best candidates. For the
applications below, list the criteria you would use to make a selection and do your best to select at least one element for the application.
A search site for properties applicable for this exercise
is:
http://www.matweb.com/
http://www.matweb.com/search/PropertySearch.aspx
Either use the first site to search for the properties of a particular element
or use the second site to fill in desired properties and search for metals that
fit the criteria. This program will find alloys as well as elements and it is ok
to list alloys instead of elements below.
There are several approaches for use of these sites to perform a search. There very well could be better approaches than those suggested here. Try to develop an approach that you are comfortable with and produces useful results. The exercises that follow do not have specific answers that are necessarily better than other answers. The primary goal is to stimulate thinking about the most important properties needed for the particular application. The elements or alloys that result should be considered informed suggestions with much more research and probably experimentation needed to to expand or narrow the list.
For specific compounds such as Plexiglas VO52i, the name can be inserted into the search box at http://www.matweb.com/ . For the exercises below, it is also possible to enter the application like "pots pans" into the search box but more options are available if you register and choose advanced search or go to: http://www.matweb.com/search/PropertySearch.aspx and choose advanced search. This enables insertion of "pots pans" into the text search and then a choice of two properties with limits. It will probably help to select "don't use wildcards." It might be worthwhile to consider limiting the material to a metal with a melting point above 600oC. Some suggestions for text entries are included with the exercises.
It might also be informative to perform a Google search such as "pots and pans composition".
| item | properties desired | best elements | image |
|
pots and pans |
 |
||
|
friendship ring |
 |
||
|
hammer head |
 |
As another example of a search for a material, consider the selection of a polymer for use as a coating on a magnetic stirring bar. Some of the properties that are needed are: high melting point [the stirring bar is sometimes used in solvents at their boiling points and melting would be very undesirable. A good starting lower limit for the melting point would be 300oC.], a low coefficient of friction (£0.03) will facilitate spinning and low water absorption (£.001%) should inhibit water penetration to the magnet. At http://www.matweb.com/search/PropertySearch.aspx , scroll down and select “Polymer”. In the boxes below, set the melting point at a minimum of 300oC (the maximum does not have to be set) and the coefficient of friction and water absorption at maxima of 0.03 and 0.001 respectively (with minima at 0). Clicking on “Find” should result in different forms of teflon or PTFE. The molded form is probably most appropriate for use as a coating for the magnet. The exercises that follow will encourage you to navigate yourself around the site to determine suitable plastics and metals and/or metal alloys for specific applications. If too many possibilities result from a search, put more severe restrictions on the properties.
C. Material selection - Polymers
item
properties desired
possible plastics
image
2 L bottle
enter "soda bottle"

frying pan coating
enter "nonstick"

telephone case
enter "telephone"

food storage
bags
enter "food bags"

tires
enter "rubber"

(For filled in table, see: III-C)
D. Material selection - Metals and/or Metal Alloys
| item | properties desired | possible metal and metal alloys | image |
| bicycle frame |  |
||
| solder |
 |
||
| electrical wire |  |
||
| nails |  |
(For filled in table, see III-D)
E. Compounds (evaluation of commercial products)
Some applications call for the selection of an organic or inorganic compound. Properties of compounds can be found Appendix A and it is possible to insert some properties into the web sites in Appendix C and have the site narrow down the list to likely candidates. The exercises below are intended primarily to focus attention on the selection of desired properties. For most of these exercises, the challenge of selecting materials is beyond the scope of this exercise. Instead some of the commercially available product ingredients are listed. Do the commercially available chemicals satisfy the properties that you considered necessary when selecting candidates for this application?
For partially filled in table, see: E.)
| Application | desirable properties | selected common ingredients | best choice |
| 1. nail polish remover | low toxicity, low dermatologic toxicity, low
cost, easy disposal, liquid or soluble in water of alcohol |
acetone, ethyl acetate, isopropyl alchohol | |
| 2, antifreeze | low mp, low toxicity, non-corrosive, low vapor pressure, low cost |
ethylene glycol, propylene glycol, glycerol | |
| 3. insect repellant | low toxicity, very low
dermatologic toxicity, repels insets, low cost, |
DEET, picaridin, IR3535, oil of lemon eucalyptus | |
| 4. antacids | very low toxicity, high efficiency, low cost | calcium carbonate, sodium bicarbonate, magnesium hydroxide, aluminum hydroxide, |
|
| 5. analgesics | low toxicity, ability to decrease pain, long lasting | acetyl salicylic acid, acetaminophen, ibuprofen), naproxen | |
| 6. sunscreen | low toxicity, very low dermatologic toxicity, opaque in near UV, low cost |
zinc oxide, titanium oxide, avobenzone, oxybenzone | |
| 7. food coloring (blue) | low toxicity,
non-carcinogenic, low cost, right absorption spectrum |
Blue No. 1 (brilliant blue), Blue No. 2 (indigotine) | |
| 8. chemicals of life |
References (selected) and notes:
2.
antifreeze
-
https://en.wikipedia.org/wiki/Antifreeze
https://www.chemicals.co.uk/blog/what-is-antifreeze
https://chem-group.com/antifreeze-the-ultimate-guide/
4. antacids - https://www.drugs.com/drug-class/antacids.html
5.
analgesics
- https://www.verywellhealth.com/what-is-the-difference-between-motrin-and-advil-770459
https://we.riseup.net/pages/infografias-data-analysis+309052/images/244134
https://www.everydayhealth.com/pain-management/which-one-should-i-take-how-to-choose-over-the-counter-pain-medication-6671
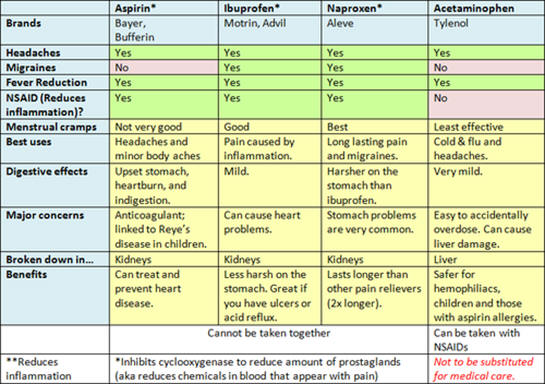
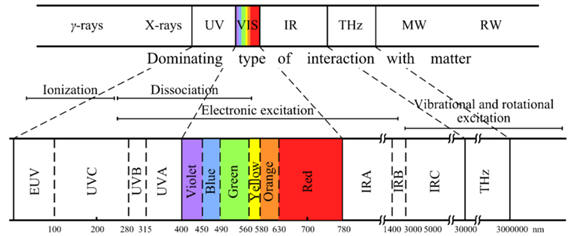
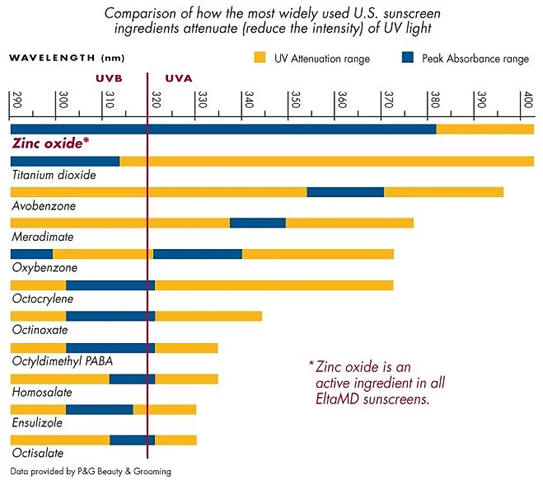
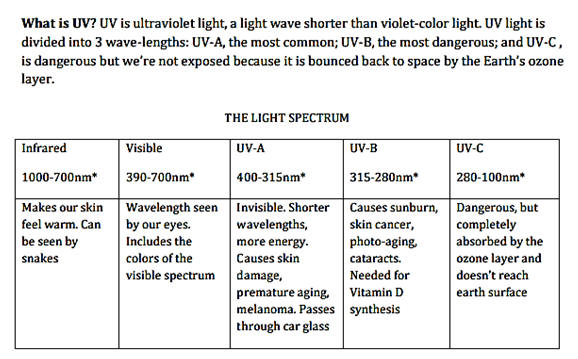 Information on radiation from the sun and compounds used as ingredients
in commercial sun screens is included above. Zinc oxide and
titanium oxide block or reflect incoming radiation while the organic
compounds are designed to absorb the radiation. In addition to the
light transmission properties, what other properties should be
considered for selection of the ingredients of sunscreen?
Information on radiation from the sun and compounds used as ingredients
in commercial sun screens is included above. Zinc oxide and
titanium oxide block or reflect incoming radiation while the organic
compounds are designed to absorb the radiation. In addition to the
light transmission properties, what other properties should be
considered for selection of the ingredients of sunscreen?
https://www.artpublikamag.com/post/when-color-kills-toxic-pigments-through-the-ages
https://newbridgewellness.com/food-dyes/
https://cspinet.org/sites/default/files/attachment/food-dyes-rainbow-of-risks.pdf
https://en.wikipedia.org/wiki/Color_of_chemicals
https://en.wikipedia.org/wiki/Aposematism
https://pubmed.ncbi.nlm.nih.gov/23026007/
8. Basic chemicals for life on earth. Three of the primary simple chemicals of life are water, oxygen and carbon dioxide. In addition organic polymers form the construction material and provide the information needed for reproduction. Is it possible that life elsewhere in the Universe could be base on different chemicals such as ammonia instead of water and silicon polymers instead of carbon polymers? Science fiction novels sometimes use the periodic table and select silicon to replace the carbon in our life systems. Consider whether options of this type could possibly sustain life. For instance, it water is going to play a significant role, are silicon bonds to itself and other elements stable in the presence of water? This is more a thought challenge but should result in thinking beyond the box and significant learning.
https://www.nbcnews.com/mach/science/silicon-based-life-may-be-more-just-science-fiction-n748266
https://en.wikipedia.org/wiki/Hypothetical_types_of_biochemistry
https://reasons.org/explore/blogs/impact-events/does-life-need-to-be-carbon-based
https://www.scienceabc.com/humans/why-is-life-on-earth-carbon-based.html
https://www.thegreatcoursesdaily.com/misconceptions-of-science-is-silicon-based-life-possible/
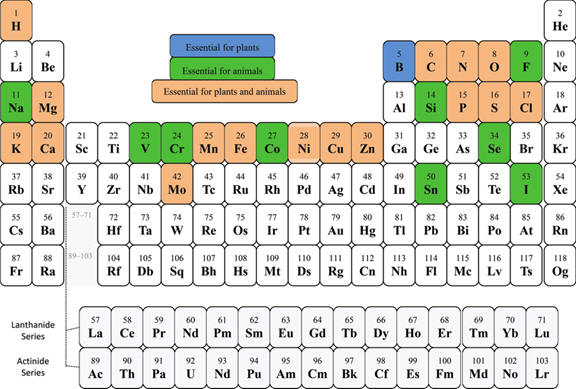
Appendix A. The properties of substances
A. Elements - Periodic Tables -
WebElements -
http://www.webelements.com/
http://murov.info/aufbaupt.htm
http://murov.info/periodictables.htm
http://murov.info/pertabtrends.htm
http://murov.info/pertabtrends.pptx
Directory
of Periodic Tables -
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All
http://murov.info/periodictables.htm
Periodic table with history of elements -
http://elements.vanderkrogt.net/
Chronology of the development of the
periodic table -
http://murov.info/timelines.htm
History of elements and periodic table -
http://www.chem.unt.edu/~jimm/REDISCOVERY%207-09-2018/
Properties of the elements -
http://www.knowledgedoor.com/
https://material-properties.org/prices-of-chemical-elements-kg/
https://dbpedia.org/page/Prices_of_chemical_elements
Appendix B.
Solvent Properties
1. Search for Compounds from
Properties Appendix D. Spectra of Substances
1. IR - Liquid or Solution
https://nickelinstitute.org/media/1771/propertiesofsomemetalsandalloys_297_.pdf
https://www.weldinghandbook.com/types-of-metals/
https://www.machinemfg.com/metal-mechanical-properties-chart/
http://www.istl.org/02-spring/internet.html
http://dol1.eng.sunysb.edu/other.html Appendix F. Solutions to Problems
B. Compounds - Properties
1.
Tabulation
Murov - http://murov.info/orgcmpds.htm
WolframAlpha - http://www.wolframalpha.com/
Chemical Book - http://www.chemicalbook.com/
Chemidplus - https://chem.nlm.nih.gov/chemidplus/ https://chem.nlm.nih.gov/chemidplus/chemidlite.jsp
Stenutz - http://www.stenutz.eu/chem/search.php
ChemBK - https://www.chembk.com/en
Knovel Critical Tables (If using Explorer, it must be
newest version)
(21824
compounds in sortable tables) - login required -
initial registration or login: https://app.knovel.com/web/register.v
already registered
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLXT1/viewerType:itble/
To
filter the listing, click on the triangle following the desired parameter,
select filter and then enter the limits of the desired parameter (<, > or =) and
hit enter.
(7274 Compounds in sortable tables) -
https://app.knovel.com/web/view/itable/show.v/rcid:kpKS000009/cid:kt00395F16/viewerType:itble/
ChemSynthesis - https://www.chemsynthesis.com/
Kaye and Laby -
organic -https://web.archive.org/web/20190519191800/http://www.kayelaby.npl.co.uk/chemistry/3_3/3_3.html
inorganic - https://web.archive.org/web/20190502165701/http://www.kayelaby.npl.co.uk/chemistry/3_2/3_2.html
Chemland 21 - http://www.chemicalland21.com/listaz01.htm
Chemblink http://www.chemblink.com/
ChemSRC - https://www.chemsrc.com/en/
LookChem
- http://www.lookchem.com/
Human
Metabolome Database - http://www.hmdb.ca/
EPA
- https://chemview.epa.gov/chemview
https://www.epa.gov/saferchoice
MolBase - http://www.molbase.com/
PubChem - https://pubchem.ncbi.nlm.nih.gov/
Osha - https://www.osha.gov/chemicaldata/
Shriner, et.al, Systematic
Identification of Organic Compounds,, manual for identifying organic
compounds
especially with the use of derivatives,
includes tables of common organic compounds with melting or
boiling points
and melting points of derivatives in tables at
back.
https://elqusb.files.wordpress.com/2018/04/kupdf-com_systematic-identification-of-organic-compounds-
wiley-shrinerhermannmorrillcurtinfuson.pdf
2.
Tabulation +
MSDS
Acros,
Fisher
-
https://www.fishersci.com/us/en/brands/I9C8LQ1I/acros-organics.html
Alpha Chemical
-
https://www.alfa.com/en/chemicals/
ChemExper Chem. Directory
- http://www.chemexper.com/
Chemidplus
-
https://chem.nlm.nih.gov/chemidplus/
https://chem.nlm.nih.gov/chemidplus/chemidlite.jsp
Chemindex - http://ccinfoweb.ccohs.ca/chemindex/search.html
Chemspider - http://www.chemspider.com/
http://www.chemspider.com/SimpleSearch.aspx
EMD Chemicals -
http://www.emdmillipore.com/US/en/documents/Z.qb.qB.tecAAAFDDJUsznLq,nav
TCI America -
http://www.tcichemicals.com/en/us/support-download/brochure/catalog.html
Wikipedia -
https://en.wikipedia.org/wiki/Dictionary_of_chemical_formulas
https://en.wikipedia.org/wiki/List_of_inorganic_compounds
Chemical Book - http://www.chemicalbook.com/
3.
MSDS or SDS
Aldrich, Sigma,-
hhttps://www.sigmaaldrich.com/united-states.html
MSDS Solutions - http://www.msds.com/
Vermont Safety Information Resources, Inc. - http://hazard.com/msds/
MSDSprovider - http://www.msdsprovider.com/
MSDS online - https://www.msdsonline.com/
MSDS digital -
https://www.msdsdigital.com/msds-database
Fisher -
https://www.fishersci.com/us/en/catalog/search/sdshome.html
EHSO - http://ehso.com/msds-sds.php
http://murov.info/orgsolvents.htm
http://murov.info/orgsolvsort.htm
https://www.organicdivision.org/ama/orig/organic_solvents.html
http://www.stenutz.eu/chem/
http://www.wiredchemist.com/chemistry/data/physical_character_solvents.html
https://www2.chemistry.msu.edu/faculty/reusch/OrgPage/solvent.htm
https://www.sigmaaldrich.com/chemistry/stockroom-reagents/learning-center/technical-library/solvent-properties.html
https://en.wikipedia.org/wiki/Solvent
https://www.ch.ic.ac.uk/williams/Solvent%20properties.pdf
Knovel Critical Tables (If using Explorer, it must be newest version)
(21824 compounds in sortable tables) - login required -
initial registration or login:
https://app.knovel.com/web/register.v
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLY34/viewerType:itble/
To filter the listing, click on the triangle
following the desirred parameter, select filter
and then enter the limits of the
desired parameter (<, > or =) and hit enter.
ChemSynthesis -
https://www.chemsynthesis.com/
nmr of deuterated
solvents
http://www.wiredchemist.com/chemistry/data/common_nmr_solvents.html
http://www2.chem.umd.edu/nmr/reference/isotope_solvent.pdf
Aldrich -
hhttp://www.sigmaaldrich.com/catalog/search/substructure/OldSubstructureSearchPage
accepts - formula, structure,
molecular mass, mp, bp, density
ChemExper Chem Directory - http://www.chemexper.com/advanced_search.shtml
accepts - formula,
structure, molecular mass, bp, mp, refractive index, density, ir,
nmr
ChemNet Data Base http://poc.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml
accepts formula, melting point, boiling point
Chemspider -
http://www.chemspider.com/FullSearch.aspx
accepts - formula, molecular mass
Melting point and
molecular mass search:
https://chem.nlm.nih.gov/chemidplus/
accepts - formula, mp,
bp
Murov - http://murov.info/orgcmpds.htm
search by molecular mass, bp, mp, density, refractiive index
Organic Chemistry Data Base - http://www.colby.edu/chemistry/cmp/cmp.html
accepts -
formula, molecular
mass, mp, bp, density, refractive index, ir, ms
NIMC site -
http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
accepts -
formula, molecular mass,
ir, nmr, ms
Polymers -
http://www.matweb.com/search/PropertySearch.aspx
Knovel Critical Tables (If using Explorer, it must be newest version)
(21824 compounds in sortable tables) - login required -
initial registration or login:
https://app.knovel.com/web/register.v
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLXT1/viewerType:itble/
To filter the listing, click on the triangle
following the desirred parameter, select filter
and then enter the limits of the
desired parameter (<, > or =) and hit enter.
(7274 Compounds in sortable tables) -
https://app.knovel.com/web/view/itable/show.v/rcid:kpKS000009/cid:kt00395F16/viewerType:itble/
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi
NIST
site - http://webbook.nist.gov/chemistry/
PSLC - http://pslc.uwsp.edu/
Aldrich,
Sigma - https://www.sigmaaldrich.com/united-states.html
Gasmet
- https://www.gasmet.com/de/products/tools/spectrum-library/
2. IR - Gas Phase
NIST - http://webbook.nist.gov/chemistry/
Gasmet
- https://www.gasmet.com/de/products/tools/spectrum-library/
3..
HNMR - Experimental
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC
- http://pslc.uwsp.edu/
Aldrich,
Sigma - https://www.sigmaaldrich.com/united-states.html
Solvents - http://www2.chem.umd.edu/nmr/reference/isotope_solvent.pdf
Deuterated solvents - http://www.wiredchemist.com/chemistry/data/common_nmr_solvents.html
bioorganics
- http://mmcd.nmrfam.wisc.edu/mmcdbrowse.html
4.
HNMR - Calculated
ChemExper
Chem Directory - http://www.chemexper.com/
nmrdb - http://www.nmrdb.org/
5. 13CNMR
- Experimental
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC
- http://pslc.uwsp.edu/
6.
Mass
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC - http://pslc.uwsp.edu/
Pherobase - http://www.pherobase.com/database/compound/compounds-index.php
human
metabolites - http://www.hmdb.ca/
7. UV-Vis
NIST site - http://webbook.nist.gov/chemistry/
OMLC - http://omlc.org/spectra/PhotochemCAD/html/alpha.html
8. Bibliography - http://library.buffalo.edu/libraries/asl/guides/spectra.html
9. Polymer spectra - http://pslc.uwsp.edu/
10. Photophysical
properties of organic compounds - http://murov.info/photophys.htm
density >20 g/cm3/strong>
melting point >3000 K
boiling point 0 to 90 K
electrical conductivity
>0.37x106 cm-1
W--1
Np, Re, Pt, Ir, Os
C/td>
He
Ag, Cu, Au, Al
Re
W
H2
Cu
Pt
Re
Ne
AAu
Ir
Os
N2
Al
Os
Ta
F2
Ar
Osub>2 (90.2K)
Element
density (g/cm3)
m.p. (oC)
hardness (Vickers)
Modulus of elasticity
(GPa)
electrical resistivity
(x106 ohm cm)
cost ($/kg)
aluminum
2.699
660.4
15
68.0
2.7
1.79
iron
7.87
1535
150
200
8.9
0.42
titanium
4.50
1660
60
116
55
11.7
III-B-1-b. The density of titanium is much lower than the density of iron but titanium
is stronger than aluminum. Unfortunately, though common in the earth's
crust, titanium is very expensive to refine. Another very important
property of titanium is that its properties have a low temperature sensitivity.
Also, unlike iron, aluminum and titanium are resistant to corrosion.
III-B-2.
| wwater absorption (% | Vical softening point (oC) | visible transmission (%) |
| 0.40 | 95 | 91 |
III-B-3-a Arkema Plexiglas HT121 Acrylic, Rohm Plexiglas GS 249 Cast Acrylic
III-B-4. Fill in desired properties and then use the matweb sites to search for
materials that fit the criteria.
| item/td> | properties desired | best materials | image |
| pots and pans enter "pots pans" |
thermal cond., cost, toxicity, tensile strength, melting point |
stainless steel, copper, aluminum, carbon steel, cast iron, stoneware |
 |
| friendship ring enter "jewelry" |
luster, cost, reactivity |
stainless steel, gold, ceramics, silver, titanium or tungsten |
 |
| hammer head enter "hammer" |
density, hardness, tensile strength, cost |
|
 |
C. Polymers
| item | properties desired | possible plastics | image |
| 2 L bottle enter "soda bottle" |
high visible transmission, low water |
PET, PE, polystyrene, polycarbonate, acrylic |
 |
| frying pan coating enter "nonstick" |
mmelting point, toxicity, cost | PTFE (teflon) |
 |
| telephone casebr /> enter "telephone" | strength, cost | ABS, PBT |
 |
| food storage bags enter "food bags" |
|
PRT, PE, PVDC |  |
| tires enter "rubber" |
|
Rubber |
 |
D. Material selection - Metals and/or Metal Alloys
| item | properties desired | possible metal and metal alloys | image |
| bicycle frame enter "bicycle", metal |
tensile strength,
elongation, cost,
density, resistance to oxidation |
titanium, aluminum, steel |  |
| ssolder enter "solder", mp |
low melting point, low elec/span>trical
resistivity |
silver, tin alloys Tin (usually the main element), Silver, Copper, Antimony, Bismuth, Cobalt, Nickel, Indium, Zinc, Germanium, and rare earth elements |
 |
| electrical wire enter "wire" electrical resistivity from 1E-06 To 1.8E-06 ohm-cm |
electrical
resistivity, melting point, cost, malleability, strength |
copper, aluminum |  |
| nails | strength, resistance
to oxidation, cost |
iron, steel |  |
E. Compounds (evaluation of commercial products)
| Application | desirable properties | selected common ingredients | Cost ($/L) | LD50 | best choice |
| 1. nail polish remover | low toxicity, low dermatologic toxicity, low
cost, easy disposal, liquid or soluble in water of alcohol |
acetone ethyl acetate isopropyl alcohol |
72 87 69 |
5,800 10,200 5,840 |
|
| 2. antifreeze | low mp, low toxicity, non-corrosive, low vapor pressure, low cost |
ethylene glycol propylene glycol glycerol |
98 180 183 |
7,712 22,000 27,200 |
|
| 3. insect repellant | low toxicity, very low
dermatologic toxicity, repels insets, low cost, |
DEET picaridin IR3535 oil of lemon eucalyptus |
80 350 100 700 |
1,892 4,743 14,000 2,480 |
|
| 4. antacids | very low toxicity, high efficiency, low cost | calcium carbonate sodium bicarbonate, magnesium hydroxide aluminum hydroxide, |
223 62 309 100 |
6,450 8,500 >2,000 |
|
| 5. analgesics | low toxicity, ability to decrease pain, long lasting |
acetylsalicylic acid acetaminophen ibuprofen naproxen |
120 268 6,000 16,800 |
1,500 1,944 1,600 534 |
|
| 6. sunscreen | low toxicity, very low dermatologic toxicity, opaque in near UV, low cost |
zinc oxide titanium oxide avobenzone oxybenzone |
128 75 74,000 77,000 |
>2,000 >10,000 >16,000 >12,800 |
|
| 7. food coloring (blue) | low toxicity,
non-carcinogenic, low cost, right absorption spectrum |
Blue No. 1 (brilliant blue) Blue No. 2 (indigotine) |
678,000 1,590 |
>1,900 2000 |
|
| 8. chemicals of life |
Note 1. During the preparation of this site, three interesting topics were encountered that did not seem to have adequate discussion during a limited Internet search.
1. Textbooks claim that except for hydrogen, isotopes have very similar properties but a comparison of physical and chemical properties of isotopes could not be located. Rate effects and nuclear effects are well documented but melting points and other physical properties of isotopes could not be found (e.g., U-235 and U-238).
2. The drug Austedo is used for the treatment of Huntington's disease. It contains deuterium atoms that apparently improve its efficacy. Although deuterium is an isotope of hydrogen, its properties are different enough that it could be considered to be a separate element but the best definitions of elements include the statement that elements have the same number of protons. This is a little like the demotion of Pluto from planet to dwarf planet.
3. Should there be a correlation between toxicity and color (visible region)?
4. A new discovery of a very strong plastic shows that the field of
material science is still in its early stages and has extensive potential
https://www.nature.com/articles/s41586-021-04296-3
,