
Experiments and Exercises for Chemistry Courses - #4
Searching for Chemicals: A Material Science Lesson for Chemistry Students. Part 1
This Exercise is #4 in Experiments and
Exercises in Chemistry Courses
(see end note 1)
For more web sites by Steven Murov including chemistry sites, jump to
http://murov.info
Textbooks written for high school and college chemistry courses contain coverage of a wide range of chemistry topics. For some purposes, less is better and it can be argued that broad coverage can result in less overall learning. A careful assessment and prioritization of topics should be performed and texts pared down to the essentials that are needed for continued learning. On the other hand, there are some very important topics that are commonly given insufficient attention. The basics of climate change and the threat posed by climate change should be included in every chemistry course (e. g., please visit: http://murov.info/climatemod.htm). Polymers, metals and metal alloys surrounds us in our daily lives but receive minimal attention in the typical chemistry course.
Chemistry laboratory experiences tend to
emphasize identification of substances and verification of identification using
properties. In contrast, very few courses
consider the challenges of the selection of materials and determination of
properties needed for various applications.
This kind of challenge can be very stimulating, instructive and provides
valuable insight into chemistry not necessarily provided by traditional
chemistry approaches. To address the shortcomings on some of these topics,
this three part lesson (see: http://murov.info/chemexpts.htm
) has been designed to provide a
brief experience with selection of materials for various applications with an
emphasis on polymers and metal alloys. The first lesson has been designed
primarily to provide familiarity with sources of information and provide
exercises on traditional problems like finding properties of compounds or
identifying compounds given some properties. The second lesson provides some examples of the search for chemicals for specific applications. This author admittedly has very
limited
expertise in the field of material science so any corrections and suggestions
will be considered and appreciated.

The discussions in this site have been divided into three categories. The first two categories are covered in many sources and are included here to provide more experience and as a review The third category (Part 2) or the search for materials based on desired properties has not received adequate exposure and is the primary purpose for the development of these sites. A Part 3 has been more recently added.
Bull and Roberts Lab, 1914 File:Bull & Roberts Lab 1914.jpg - Wikimedia Commons
CONTENTS
I. The determination of properties of substances
II. Identification of substances from properties.
III. Searches for materials based on desired properties. Jump to http://murov.info/matsci2.htm
Appendix A. The Properties of Substances (Elements, Compounds)
Appendix B. The Properties of Solvents
Appendix C. Search for Compounds from Properties
Appendix D. Spectra of Substances
Appendix E. Links to Information about Metal Alloys
Appendix F. Solutions to Problems
I. The determination of properties of substances
Given a substance, there are many
approaches that can be used to determine the literature values of the properties
of the substance. A couple of decades ago, the starting point was often
the Handbook of Chemistry and Physics, the Dictionary of Organic
Compounds or the Merck Index. While these resources are
still valuable sources of information, the three books mentioned are large and
cumbersome to carry around. With smart phone access to the Internet, it is
often possible to find properties very quickly using one of several available web
sites. Appendix A contains hot links to many of these
sites. However, use all
Internet sites with caution as many contain a huge collection of data and errors
are not uncommon. The exercises in this section have been designed to
provide an introductory experience with some of the sites that provide
properties of substances. For the first section on elements, any of the
many periodic table sites often will provide sufficient information. For
the properties of compounds, experience with the sites listed in
Appendix A is the best
educational pathway to acquire an understanding of the usefulness of the sites.
A. Properties of Elements
1. Physical Data. In 1869 when Dimitri Mendeleev published a periodic
table that has survived over 150 years, only 63 of the currently discovered elements
(118) had been discovered. Mendeleev arranged the elements according to
increasing atomic mass but started new groups with each of the alkali metals as
he found that in doing so, the elements in vertical columns had properties
similar to the other elements in the group (e.g., lithium, sodium, potassium,
rubidium and cesium all react violently with water to produce hydrogen gas and a
basic solution). To sort the elements according to reactivity properties,
Mendeleev brilliantly left spaces in his chart for elements not yet discovered
so that elements that followed aligned
with similar elements above and below them. For two of the empty element spaces,
Mendeleev confidently named the elements eka aluminum and eka silicon after the
elements above the spaces in his table. See the image below. Based
on the properties of the surrounding elements, Mendeleev predicted properties
for eka-aluminum and eka-silicon (see table below). Fill in the missing spaces in the table and
comment on the accuracy of Mendeleev's predictions.
Useful resources:
http://www.knowledgedoor.com/
https://www.webelements.com/
(under binary compounds)

|
Property |
Eka- |
|
Eka-silicon |
|
|
Atomic mass (g/mole |
68 |
|
70 |
|
|
Density (g/cm3) |
6.0 |
|
5.5 |
|
|
Melting point |
low |
|
high |
|
|
Oxide formula |
Ea2O3 |
|
|
|
|
Chloride formula |
EaCl3 |
|
EsCl4 |
|
2. Properties of iodine. As mentioned, Mendeleev arranged the elements according to increasing atomic mass (it was not until over 3 decades later that the partial composition of the atom was understood and it was determined that the number of protons in the nucleus determines the arrangement rather than the atomic mass) but noticed that this resulted in some incorrect alignments including the positions of tellurium and iodine. He placed them correctly despite the reversal in their atomic mass values and attributed the problem to inaccurate atomic mass measurements. Name some properties of iodine that probably led Mendeleev to place iodine below bromine.
3.
 Properties of oxygen: Fill in the table for the properties of
oxygen.
Properties of oxygen: Fill in the table for the properties of
oxygen.
(filled in table I-A-3)
| Property | boiling point (K) | melting point (K) | allotropes | Lewis structures of O2 |
a.
The image shows the pouring of liquid oxygen through a magnetic field. a.
What do you observe?
(Answer at II-A-3-a)
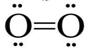
b. The Lewis structure to the right satisfies the rules for the drawing of a Lewis structure. Does the structure explain the behavior of liquid oxygen in a magnetic field. If not, is it possible to draw a Lewis structure for oxygen that follows Lewis structure guidelines? (Answer I-A-3-b))
B. Properties of Compounds (and a couple of elements): 1. For each of the substances in the table, fill in the property of all spaces that do not have an X in them. The substances are arranged in alphabetical order but questions that follow the table focus on pairs or groups of substances that might be separated in the table. Following the table is a series of questions about the compounds in the table. The questions are intended to stimulate interest in fascinating properties of substances. Many of these substances have previously been selected as a molecule of the month http://www.chm.bris.ac.uk/motm/motm.htm. For a Powerpoint presentation on fascinating chemicals, please visit: http://murov.info/FascinatingChemicals.ppt . (filled in table I-B)
| Compound/Property | Formula | molecular mass (g/mole) |
common name | m.p. (oC) | b.p. (oC) | density (solid) (g/cm3) | density (liquid) (g/cm3) | LD50 |
| acetic acid | X | X | ||||||
| acetaminophen | tylenol | X | X | X | ||||
| amoxicillin | X | X | X | |||||
| d-carvone | X | X | X | |||||
| l-carvone | X | X | X | |||||
| dideuterium monoxide | heavy water | -- | X | |||||
| dihydrogen monoxide | water | at OoC | X | |||||
| dioxin | X | X | X | |||||
| nitrogen dioxide | X | X | X | X | ||||
| penicillin G | X | X | ||||||
| 11-cis-retinal | X | X | X | |||||
| all trans-retinal | X | X | X | |||||
| styrene | X | X | X | |||||
| 2,2,4-trimethylpentane | isooctane | X | X | |||||
| uranium -235 | X | X | ||||||
| uranium-238 | X | X | ||||||
| vanillin | X | X | X |
While there is overlap, the questions below have been divided into several categories: physical properties, chemical properties, isotopes, isomers and toxicity.
 Physical properties - The difference
between the density of the liquid and solid states of a substance at its melting
point. The photo to the right shows three substances, each at their
melting (or freezing temperature) point. The three substances in
alphabetical order are acetic acid, water and
p-xylene.
Physical properties - The difference
between the density of the liquid and solid states of a substance at its melting
point. The photo to the right shows three substances, each at their
melting (or freezing temperature) point. The three substances in
alphabetical order are acetic acid, water and
p-xylene.
2. Which of three is water? (I-B-2 for answer)
Amazingly, of the millions of known substances, water is probably the only substance where you have observed the solid and liquid in a container and probably accept its behavior as normal. But think about this situation: as the temperature drops to the freezing point, should the liquid expand or contract (should the density increase with decreasing temperature or increase?). Hopefully, you responded that the volume of the liquid should decrease with cooling resulting in an increase in density (density = mass/volume). A decrease in volume increases the density as the mass does not change. If this contraction continues with freezing, the solid should have a higher density than the liquid and it should sink in its liquid state. This is the expected behavior and it is realized like the 2nd and 3rd test tubes for almost all substances except for water. The conclusion then is that water must be in the first tube since its density behavior is very uncommon resulting in the ice floating in liquid water.
3. If water behaved like almost all other substances, what would happen to lakes in freezing weather? (I-B-3 for ans.)
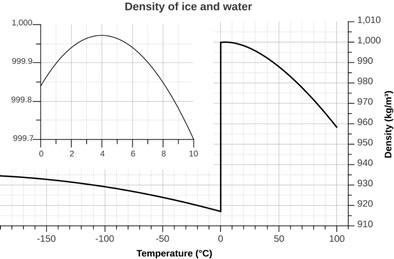
4. The behavior of the density
of states of water as a function of temperature is shown in the graph below
Suggest a reason for the unusual behavior of the density of water at its melting
point. http://murov.info/EM-st1.pdf, Murov,
S. J. Chem. Ed., 2013, 90,
1484-5. "Liquid-Solid
Density: Observations and Demonstrations." (Cover)
http://pubs.acs.org/toc/jceda8/90/11
https://pubs.acs.org/doi/10.1021/ed300162x
.
(I-B-4 for
answer)
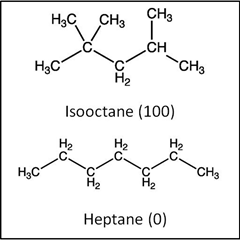
C. Chemical properties. Octane ratings for automotive gasoline are often based on
arbitrarily setting the "knocking"
performance of
2,2,4-trimethylpentane at 100 and n-heptane at 0.
Knocking occurs when the combustion rate of the gasoline is not optimal for
the engine. 100 is generally good and 0 means a lot of knocking. If
the octane of a sample is rated 87, it knocks at the same rate as an
87:13 mixture of 2,2,4-trimethylpentane and n-heptane.
Prefix iso is
used for those isomeric (same formula) alkanes in which one methyl group is
attached to the next to end carbon atom (second last) of the continuous chain.
Using
this system, 2-methylheptane can be called iso-octane. The fuel industry
has named 2,2,4-trimethylpentane "isooctane".
1. Is this proper use of the
prefix iso and if not, what is the problem with this use?
(I-C-1 for answer)
As a result of polymer chemistry, we are surrounded by objects made out of many different kinds of plastics. In
the 1967 classic movie, The Graduate, the following conversation was
heard between the main character, Benjamin Braddock (portrayed by Dustin
Hoffman, a 29 year-old playing a 21 year-old in his film debut), the guests and
a family friend, Mr. McGuire, at Ben’s college graduation party:
Guests: We're all so proud of you, proud, proud,
proud, proud, proud, proud, proud. What are you going to do now?
Ben: I was going to go upstairs for a minute.
Guests: I meant with your future, your life.
Ben: Well, that's a little hard to say.
Mr. McGuire: I just want to say one word to you - just one word.
Ben: Yes sir.
Mr. McGuire: Are you listening?
Ben: Yes I am.
Mr. McGuire: 'Plastics.'
Ben: Exactly how do you mean?
Mr. McGuire: There's a great future in plastics. Think about it. Will you think
about it?
Ben: Yes I will.
Mr. McGuire: Shh! Enough said. That's a deal.

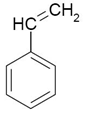 Styrofoam is one of the many commonly used plastics. Among many uses, it
is found in fast food containers and shipping containers. Styrofoam is a
form of polystyrene and is made by polymerizing styrene. The
essential part of styrene for the polymerization process is the C=C double bond.
If a hydrogen is in the place of the phenyl (benzene ring), polyethylene is the
resulting polymer and if the benzene ring is replaced by a methyl,
Styrofoam is one of the many commonly used plastics. Among many uses, it
is found in fast food containers and shipping containers. Styrofoam is a
form of polystyrene and is made by polymerizing styrene. The
essential part of styrene for the polymerization process is the C=C double bond.
If a hydrogen is in the place of the phenyl (benzene ring), polyethylene is the
resulting polymer and if the benzene ring is replaced by a methyl,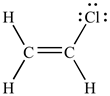 polypropylene
is the resulting polymer.
polypropylene
is the resulting polymer.
2. If the benzene ring is replaced by a chlorine, draw a short segment of the resulting polymer that can be formed and name some of its uses. (I-C-2 for answer)
 3, Nitrogen dioxide is one of the dangerous pollutants in smog. In an
internal combustion engine, air introduced causes the reaction (combustion) of
oxygen with gasoline. Air is 78% nitrogen, 21% oxygen and almost 1% argon.
The heat of combustion raises the temperature in the cylinder to the point that
the oxygen can react with nitrogen producing nitrogen monoxide (nitric oxide).
The nitric oxide eventually reacts with more oxygen to produce NO2.
3, Nitrogen dioxide is one of the dangerous pollutants in smog. In an
internal combustion engine, air introduced causes the reaction (combustion) of
oxygen with gasoline. Air is 78% nitrogen, 21% oxygen and almost 1% argon.
The heat of combustion raises the temperature in the cylinder to the point that
the oxygen can react with nitrogen producing nitrogen monoxide (nitric oxide).
The nitric oxide eventually reacts with more oxygen to produce NO2.
a. Write equations for the formation of NO and NO2 and draw a
Lewis structure for NO2. (I-C-3-a for
answer)
c. Given the opportunity, NO2 tends to partially dimerize to N2O4.
Draw the Lewis structure of N2O4 including formal charges
and suggest an explanation for the dimerization not going to completion.
(I-C-3-c
for answer)
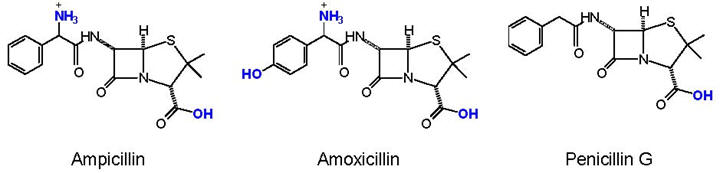
4. The three penicillin structures shown above are amoxicillin, ampicillin and penicillin G. Penicillin G was serendipitously discovered by Alexander Fleming in 1928 but it was not until the urgency of WWII infections that a method for delivering penicillin was developed. Unfortunately, penicillin G is not stable in stomach acid and has to be injected to be effective. While injections are vastly superior to no treatment for bacterial infections, chemists and pharmacologists were successful with their research efforts to modify the penicillin structure so that it would be stable in stomach acid. Amoxicillin and ampicillin are two of the results of the research and are still commonly used today to treat infections.
a. What change common to both molecules was made to penicillin G to make amoxicillin and ampicillin? (I-C-4-a for answer).
b. Why does this change make the penicillin derivatives stable in acid?
(I-C-4-b for answer)
c. Could the researchers have been sure that the structural change would
not decrease antibiotic activity? Explain your answer.
(I-C-4-c
for answer)
D. Isotopes - The number of protons in the nucleus of an atom determine the identity of an element. If, by some nuclear method, the number of protons is changed, a different element results. For the first 20 elements, the number of neutrons is close to the number of neutrons but after atomic number 20, the average number of neutrons added with each additional proton is greater than one. When uranium is reached, there are 92 protons and 146 neutrons. For many elements and especially the even atomic numbered elements, different numbers of neutrons can be present and still result in stable nuclei. Nuclei with the same number of protons but different numbers of neutrons are called isotopes. Because isotopes differ primarily in mass, physically and chemically, isotopes behave almost the same. However, for the lighter elements and especially hydrogen, reactions directly involving the isotope can significantly affect the rates of reaction. This explainable observation can be used as a technique to explore mechanisms of reaction. On the other hand, isotopes can differ significantly in their nuclear properties. Except for limited proton to neutron ratios, most combinations do not result in stable nuclei and the nuclei are radioactive. Other nuclear properties such as fission and fusion are also very isotope dependent.
1. Consider the properties of water and heavy water. Are there significant differences and are the differences explainable from isotopic differences? Is the density difference consistent with the mass difference? (I-D-1 for answer)
2.
Do you think it would be safe to drink heavy water? (I-D-2
for answer)
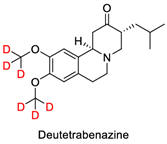
As a point of interest, there have been efforts to improve drug
efficacy by replacing hydrogen atoms with deuterium atoms. Bonds to
deuterium break up to several times slower than bonds to hydrogen.
Deuterium substitution could result in longer drug life and action and possibly
decreased side effects. The result of this research has resulted in FDA
approval of deutdetrabenazine (commercially called Austedo
(used
to decrease involuntary movements (chorea) caused by Huntington's disease).
https://www.dovepress.com/review-of-deutetrabenazine-a-novel-treatment-for-chorea-associated-wit-peer-reviewed-fulltext-article-DDDT
https://pubs.acs.org/doi/10.1021/acs.biochem.7b00765
https://pubmed.ncbi.nlm.nih.gov/25069517/
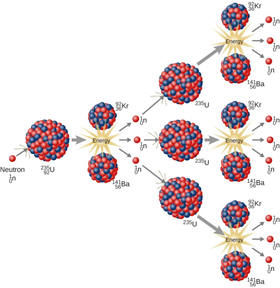
The two primary isotopes of uranium found on earth are shown below along with the nuclear reactions for radioactive decay. U-235 undergoes fission when impacted by neutrons and is used in nuclear reactors and bombs. U-238 does not undergo fission but can be converted to fissionable Pu-239. The problem is that U-238 composes 99.7% of naturally occurring uranium. For uranium to be fissionable, it must contain much higher than 0.3% U-235. Because isotopes have very similar physical and chemical properties, it is difficult and expensive to enrich uranium enough to make it fissionable. The enrichment process was one of the reasons for the Iranian treaty which regulated the amount of enrichment. However, the U.S. pulled out of the treaty giving Iran more leeway in enrichment.
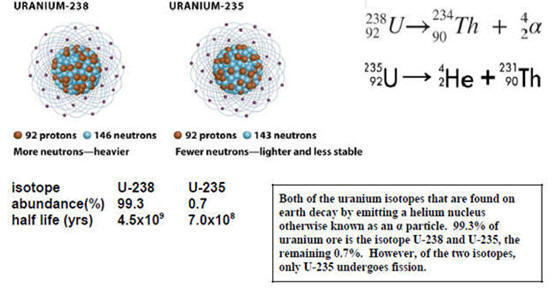
3. Write a nuclear reaction for the fission of U-235. (I-D-3 for answer)
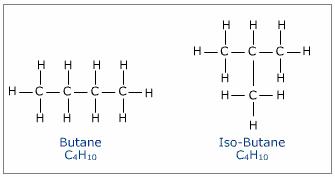
E. Isomers are compounds with the same formula but differ structurally in some way. For instance, 2-methylpropane (isobutane) is a structural isomer of n-butane. Because of the structural differences, the chemical and physical properties of isomers usually differ sometimes substantially. Stereoisomers are not necessarily easy to distinguish but play a very important role in nature.
Geometric isomers are a type of stereoisomer that usually differ by the
orientation of the groups attached to a double bond. The figure to the
right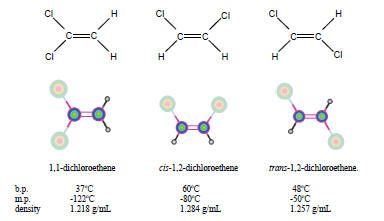 show the geometric isomers (2nd and 3rd structures) of dichlorethane and
also a structural isomer.
Because rotation around a double bond does not occur at room temperature, the
geometric isomers can be distinguished and separated. One very important
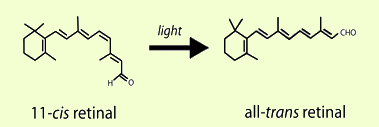
application of this 11-cis trans isomerism occurs in the eye and
enables vision. Energy from light causes 11-cis retinal to
isomerize to all-trans retinal resulting in a signal to the brain that
results in vision.
show the geometric isomers (2nd and 3rd structures) of dichlorethane and
also a structural isomer.
Because rotation around a double bond does not occur at room temperature, the
geometric isomers can be distinguished and separated. One very important
application of this 11-cis trans isomerism occurs in the eye and
enables vision. Energy from light causes 11-cis retinal to
isomerize to all-trans retinal resulting in a signal to the brain that
results in vision.
1. Use an analogy to a lock and key to explain how
conversion of
11-cis retinal to all-trans retinal could result
in rejection of the key from the lock. (I-E-1 for answer)
An even more subtle form of stereoisomerism occurs when two compounds are mirror
images that are not superimposable. There are many extremely important
examples in nature where this type of isomerism occurs including the sugar
family and the amino acids used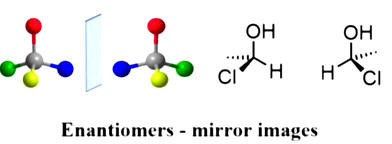 to form polypeptides and proteins.
Non-superimposable mirror images have many identical physical properties but
differ in the way they rotate polarized light and importantly, the way they
interact with other stereoisomers.
to form polypeptides and proteins.
Non-superimposable mirror images have many identical physical properties but
differ in the way they rotate polarized light and importantly, the way they
interact with other stereoisomers.
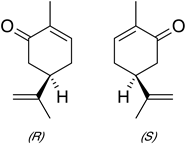
An example that you might have encountered is that the primary components of caraway and spearmint oil are d-carvone and l-carvone respectively that are mirror images of each other. The two isomers fit into different sites in the nose resulting in different odors perceived by the brain with d-carvone giving a caraway odor and l-carvone a spearmint odor.
2. Compare the properties of the two carvones and comment on
similarities and differences. Are the differences experimental or real?
For more information, please visit:
S. L. Murov and M. Pickering, J.
Chem. Ed., 50,
74 (1973). "The Odor of Optical Isomers - An Experiment in Organic Chemistry."
https://pubs.acs.org/doi/10.1021/ed050p74
(I-E-2 for answer)
F. Toxicity.
1. For the compounds listed, record the oral LD50 values
for rats and calculate the amount that would kill half the population of 70 kg
humans assuming that the extrapolation is valid.
(source
Appendix A-2-b. I-F-1 for answer)
| Compound | oral LD50 value for rats | extrapolated amount for 70 kg human |
| acetaminophen | ||
| dioxin | ||
| vanillin |
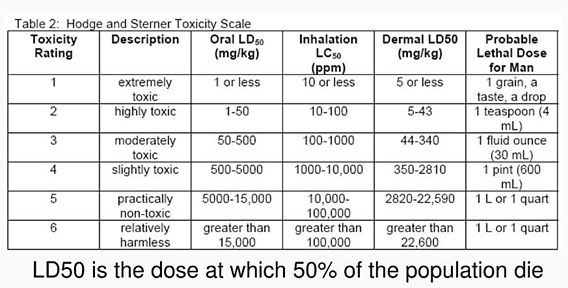
https://www.slideserve.com/noelani/environmental-hazards-and-human-health slide 37
2. Would you consider vanillin to be a safe food additive? Explain your answer. (I-F-2 for answer)
3. Acetaminophen is the active ingredient in “non-aspirin” pain relievers. Extra strength tablets contain 500 mg of acetaminophen. How many tablets would be toxic to half the population of 70 kg humans (assuming the rat LD50 values apply to humans)? (I-F-3 for answer)
4.
https://www.slideserve.com/noelani/environmental-hazards-and-human-health slide 37
Dioxin is a by product of bleached paper production. For a disaster
involving dixoin, please visit:
https://www.theatlantic.com/photo/2014/12/bhopal-the-worlds-worst-industrial-disaster-30-years-later/100864/. Often environmentalists urge the replacement of plastic food plates
with paper plates. Is this clearly a good way forward? Explain your
answer. (I-F-4 for answer)
5. The venom of black widows has a rat LD50 of approximately 0.002 mg/kg and rattlesnake venom has an LD50 of about 0.5 mg/kg. Assuming a density of 1 g/mL, what volume of venom do black widows and rattlesnakes have to inject to have a 50% chance of killing a 70 kg human? What are the many assumptions that go into this calculation? Does the amount intuitively seem like a reasonable amount for a spider bite? (I-F-5 for answer)
See:
https://en.wikipedia.org/wiki/Pathophysiology_of_spider_bites
https://pubmed.ncbi.nlm.nih.gov/3775789/
https://untamedscience.com/blog/worlds-deadliest-snakes/
https://biochem-molbio.imedpub.com/determination-of-lethal-dose-ld50of-venom-of-four-different-poisonoussnakes-found-in-pakistan.php?aid=21045
II. Identification of substances from properties.
The sites in this section includer problem solving experience by providing
physical properties, ir, nmr and or mass spectra. This is a small selection of those available on the
Internet. There are also many instructional sites available that teach students
how to solve problems and interpret spectra. Searches for instructional
sites are left to the user. The first part of the exercise involves the
solving of unknowns given some physical properties and limited spectral
information. Appendix C has several sites that can be used to assist with
this assignment. Inputting the information into the sites will yield
possible hits. It is sometimes easier to solve these challenges without
the assistance of the
Appendix C sites.
Searching for Unknowns. Use one or more of the sites (this part of the exercise
was designed for use with the Organic Chemistry Data Base (https://www.colby.edu/chemistry/cmp/cmp.html)
from Appendix C to find the best matches to the information provided in
each question below. Of the sites in Appendix C, it is the best for inputting
refractive index information. The site,
http://murov.info/orgcmpds.htm also
includes refractive indices and has sortable columns. The possible
compounds list that results depends on which web site is used. Those listed
in the answers are results from
the Organic Chemistry Data Base. For the three cases
where there are multiple possibilities, suggest a method for distinguishing
among them.
(Filled in table
at II.)
| # | m.p. (oC) | b.p. (oC) | refractive index | IR info | Possible Cmpds. | Suggestions |
| 1 | 115 | 1.4100 | -OH, -CH3 | |||
| 2 | 122 | -OH, C=O | ||||
| 3 | 154 | 1.5160 | arom., -CH3 | |||
| 4 | 202 | 1.5325 | C=O, -CH3 | |||
| 5 | 81 | arom., -CH3 | ||||
| 6 | 78 | 1.373 | -CH3, C=O | |||
| 7 | 114 | arom., C=O, -NH, -CH3 | ||||
| 8 | 134 | 1.4585 | sp3 C-H |
For more exercises with many spectra included, please visit:
http://murov.info/orglab/51-x5.pdf
http://www3.nd.edu/~smithgrp/structure/workbook.html
https://www.orgchemboulder.com/Spectroscopy/Problems/index.shtml
https://webspectra.chem.ucla.edu/
http://chemistry.miamioh.edu/organicspecid/
http://alpha.lasalle.edu/~price/202 COMBINED SPECTROSCOPY PROBLEMS.pdf
Appendix A. The properties of substances
1. Elements -
Periodic Tables -
WebElements -
http://www.webelements.com/
http://murov.info/aufbaupt.htm
http://murov.info/periodictables.htm
http://murov.info/pertabtrends.htm
http://murov.info/pertabtrends.pptx
Directory
of Periodic Tables -
https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All
http://murov.info/periodictables.htm
Periodic table with history of elements -
http://elements.vanderkrogt.net/
Chronology of the development of the
periodic table -
http://murov.info/timelines.htm
History of elements and periodic table -
http://www.chem.unt.edu/~jimm/REDISCOVERY%207-09-2018/
Properties of the elements -
http://www.knowledgedoor.com/
https://material-properties.org/prices-of-chemical-elements-kg/
https://dbpedia.org/page/Prices_of_chemical_elements
2. Compounds - Properties
a. Tabulation
Murov - http://murov.info/orgcmpds.htm
WolframAlpha - http://www.wolframalpha.com/
Chemical Book - http://www.chemicalbook.com/
Chemidplus - https://chem.nlm.nih.gov/chemidplus/ https://chem.nlm.nih.gov/chemidplus/chemidlite.jsp
Stenutz - http://www.stenutz.eu/chem/search.php
ChemBK - https://www.chembk.com/en
Knovel Critical Tables (If using Explorer, it must be
newest version)
(21824
compounds in sortable tables) - login required -
initial registration or login: https://app.knovel.com/web/register.v
already registered
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLXT1/viewerType:itble/
To
filter the listing, click on the triangle following the desirred parameter,
select filter and then enter the limits of the desired parameter (<, > or =) and
hit enter.
(7274 Compounds in sortable tables) -
https://app.knovel.com/web/view/itable/show.v/rcid:kpKS000009/cid:kt00395F16/viewerType:itble/
ChemSynthesis - https://www.chemsynthesis.com/
Kaye and Laby -
organic -https://web.archive.org/web/20190519191800/http://www.kayelaby.npl.co.uk/chemistry/3_3/3_3.html
inorganic - https://web.archive.org/web/20190502165701/http://www.kayelaby.npl.co.uk/chemistry/3_2/3_2.html
Chemland 21 - http://www.chemicalland21.com/listaz01.htm
Chemblink http://www.chemblink.com/
ChemSRC - https://www.chemsrc.com/en/
LookChem
- http://www.lookchem.com/
Human
Metabolome Database - http://www.hmdb.ca/
EPA
- https://chemview.epa.gov/chemview
https://www.epa.gov/saferchoice
MolBase - http://www.molbase.com/
PubChem - https://pubchem.ncbi.nlm.nih.gov/
Osha - https://www.osha.gov/chemicaldata/
Shriner, et.al, Systematic
Identification of Organic Compounds, manual for identifying organic
compounds especially
with the use of derivatives, includes tables of common organic compounds with melting or
boiling points
and melting points of derivatives in tables at
back.
https://celqusb.files.wordpress.com/2018/04/kupdf-com_systematic-identification-of-organic-compounds-wiley-shrinerhermannmorrillcurtinfuson.pdf
b..
Tabulation +
MSDS
Acros,
Fisher
-
https://www.fishersci.com/us/en/brands/I9C8LQ1I/acros-organics.html
Alpha Chemical
-
https://www.alfa.com/en/chemicals/
ChemExper Chem. Directory
- http://www.chemexper.com/
Chemidplus
-
https://chem.nlm.nih.gov/chemidplus/
https://chem.nlm.nih.gov/chemidplus/chemidlite.jsp
Chemindex - http://ccinfoweb.ccohs.ca/chemindex/search.html
Chemspider - http://www.chemspider.com/
http://www.chemspider.com/SimpleSearch.aspx
EMD Chemicals -
http://www.emdmillipore.com/US/en/documents/Z.qb.qB.tecAAAFDDJUsznLq,nav
TCI America -
http://www.tcichemicals.com/en/us/support-download/brochure/catalog.html
Wikipedia -
https://en.wikipedia.org/wiki/Dictionary_of_chemical_formulas
https://en.wikipedia.org/wiki/List_of_inorganic_compounds
Chemical Book - http://www.chemicalbook.com/
c.
MSDS or SDS
Aldrich, Sigma,-
https://www.sigmaaldrich.com/united-states.html
MSDS Solutions - http://www.msds.com/
Vermont Safety Information Resources, Inc. - http://hazard.com/msds/
MSDSprovider - http://www.msdsprovider.com/
MSDS online - https://www.msdsonline.com/
MSDS digital -
https://www.msdsdigital.com/msds-database
Fisher -
https://www.fishersci.com/us/en/catalog/search/sdshome.html
EHSO - http://ehso.com/msds-sds.php
http://murov.info/orgsolvents.htm
http://murov.info/orgsolvsort.htm
https://www.organicdivision.org/ama/orig/organic_solvents.html
http://www.stenutz.eu/chem/
http://www.wiredchemist.com/chemistry/data/physical_character_solvents.html
https://www2.chemistry.msu.edu/faculty/reusch/OrgPage/solvent.htm
https://www.sigmaaldrich.com/chemistry/stockroom-reagents/learning-center/technical-library/solvent-properties.html
https://en.wikipedia.org/wiki/Solvent
https://www.ch.ic.ac.uk/williams/Solvent%20properties.pdf
Knovel Critical Tables (If using Explorer, it must be newest version)
(21824 compounds in sortable tables) - login required -
initial registration or login:
https://app.knovel.com/web/register.v
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLY34/viewerType:itble/
To filter the listing, click on the triangle
following the desirred parameter, select filter
and then enter the limits of the
desired parameter (<, > or =) and hit enter.
ChemSynthesis -
https://www.chemsynthesis.com/
nmr of deuterated
solvents
http://www.wiredchemist.com/chemistry/data/common_nmr_solvents.html
http://www2.chem.umd.edu/nmr/reference/isotope_solvent.pdf
Appendix C.
Search for Compounds from Properties
Aldrich -
http://www.sigmaaldrich.com/catalog/search/substructure/OldSubstructureSearchPage
accepts - formula, structure,
molecular mass, mp, bp, density
ChemExper Chem Directory - http://www.chemexper.com/advanced_search.shtml
accepts - formula,
structure, molecular mass, bp, mp, refractive index, density, ir,
nmr
ChemNet Data Base http://poc.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml
accepts formula, melting point, boiling point
Chemspider -
http://www.chemspider.com/FullSearch.aspx
accepts - formula, molecular mass
Melting point and
molecular mass search:
https://chem.nlm.nih.gov/chemidplus/
accepts - formula, mp,
bp
Murov - http://murov.info/orgcmpds.htm
search by molecular mass, bp, mp, density, refractiive index
Organic Chemistry Data Base - http://www.colby.edu/chemistry/cmp/cmp.html
accepts -
formula, molecular
mass, mp, bp, density, refractive index, ir, ms
NIMC site -
http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
accepts -
formula, molecular mass,
ir, nmr, ms
Polymers -
http://www.matweb.com/search/PropertySearch.aspx
Knovel Critical Tables (If using Explorer, it must be newest version)
(21824 compounds in sortable tables) - login required -
initial registration or login:
https://app.knovel.com/web/register.v
https://app.knovel.com/web/login.v
,
click on or copy and paste:
https://app.knovel.com/web/view/itable/show.v/rcid:kpKCTE000X/cid:kt002VLXT1/viewerType:itble/
To filter the listing, click on the triangle
following the desirred parameter, select filter
and then enter the limits of the
desired parameter (<, > or =) and hit enter.
(7274 Compounds in sortable tables) -
https://app.knovel.com/web/view/itable/show.v/rcid:kpKS000009/cid:kt00395F16/viewerType:itble/
Appendix D. Spectra of Substances
1. IR - Liquid or Solution
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi
NIST
site - http://webbook.nist.gov/chemistry/
PSLC - http://pslc.uwsp.edu/
Aldrich,
Sigma - https://www.sigmaaldrich.com/united-states.html
Gasmet
- https://www.gasmet.com/de/products/tools/spectrum-library/
2. IR - Gas Phase
NIST - http://webbook.nist.gov/chemistry/
Gasmet
- https://www.gasmet.com/de/products/tools/spectrum-library/
3..
HNMR - Experimental
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC
- http://pslc.uwsp.edu/
Aldrich,
Sigma - https://www.sigmaaldrich.com/united-states.html
Solvents - http://www2.chem.umd.edu/nmr/reference/isotope_solvent.pdf
Deuterated solvents - http://www.wiredchemist.com/chemistry/data/common_nmr_solvents.html
bioorganics
- http://mmcd.nmrfam.wisc.edu/mmcdbrowse.html
4.
HNMR - Calculated
ChemExper
Chem Directory - http://www.chemexper.com/
nmrdb - http://www.nmrdb.org/
5. 13CNMR
- Experimental
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC
- http://pslc.uwsp.edu/
6.
Mass
NIMC site - http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
PSLC - http://pslc.uwsp.edu/
Pherobase - http://www.pherobase.com/database/compound/compounds-index.php
human
metabolites - http://www.hmdb.ca/
7. UV-Vis
NIST site - http://webbook.nist.gov/chemistry/
OMLC - http://omlc.org/spectra/PhotochemCAD/html/alpha.html
8. Bibliography - http://library.buffalo.edu/libraries/asl/guides/spectra.html
9. Polymer spectra - http://pslc.uwsp.edu/
10. Photophysical
properties of organic compounds - http://murov.info/photophys.htm
Appendix E. Links to Information about metal alloys
https://nickelinstitute.org/media/1771/propertiesofsomemetalsandalloys_297_.pdf
https://www.weldinghandbook.com/types-of-metals/
https://www.machinemfg.com/metal-mechanical-properties-chart/
http://www.istl.org/02-spring/internet.html
http://dol1.eng.sunysb.edu/other.html
Appendix F. Solutions to Problems
|
Property |
Eka- |
|
Eka |
|
|
Atomic mass (g/mole |
68 |
|
70 |
|
|
Density (g/cm3) |
6.0 |
|
5.5 |
|
|
Melting point |
low |
|
high |
|
|
Oxide formula |
Ea2O3 |
|
|
|
|
Chloride formula |
EaCl3 |
|
EsCl4 |
GeCl2, GeCl4 |
Mendeleev's predictions were very close and made the discoveries of gallium and germanium easier as the researchers had a good idea of what to look for.
I-A-2. (Iodine) Like all of the halogens, the most common oxidation state of iodine in compounds is -1 and they form 1:1 compounds (e.g. KI) with the alkali metals. Tellurium properties are not consistent with those of the other halogens. The halogens behave as non-metals but tellurium has both metal and non-metal properties.
I-A-3. Properties of oxygen: Filled in the table for the properties of oxygen.
| Property | boiling point (K) | melting point (K) | allotropes | Lewis structures of O2 |
| 90.2 | 54.4 | O, O2, O3, O4 atomic oxygen, dioxygen, ozone, tetraoxygen |  |
I-A-3-a. The image shows that the liquid oxygen bends towards one of the magnetic poles.
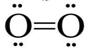
I-A-3-b. The first Lewis structure of O2 shown fits all the Lewis structure guidelines. The second one does not satisfy the octet rule and therefore would not be favored as the correct structure since the first structure does satisfy the rules. However, the first structure has all paired electrons and would not result in the magnetic behavior observed. The second structure does have unpaired electrons and is more consistent with the observation. This is a very rare case where the favored Lewis structure is apparently not a good model for the molecule.
| Compound/Property | Formula | molecular mass (g/mole) |
common name | m.p. (oC) | b.p. (oC) | density (sold) (g/cm3) | density (liquid) (g/cm3) 25oC | refractive index | LD50 (mg/Kg) |
| acetic acid | C2H4O2 | 60.05 | X | 16.6 | 117.9 | 1.26 (17oC) | 1.049 | 1.2717 | X |
| acetaminophen |
|
151.2 | tylenol | 168 | 420 | 1.293 | X | X | 1944 |
| amoxicillin |
| 365,41 | X | 194 | 743 | 1.6 | X | 1.702 | >1.5x104 |
| d-carvone (S) | C10H14O | 150,22 | X | 25.2 | 231 | X | 0.963 | 1.497 | X |
| l-carvone (R) | C10H14O | 150.22 | X | 25.2 | 231 | X | 0.963 | 1.497 | X |
| dideuterium monoxide | D2O | 20.03 | heavy water | 3.8 | 101.4 | 1.106 (11.06oC) | 1.107 | 1.328 | X |
| dihydrogen monoxide | H2O | 18.02 | water | 0.00 | 100.00 | 0.916 (0oC) | 0.998 | 1.333 | X |
| dioxin |
|
321.97 | X | 305 | X | 1.8 | X | 1.437 | 2x10-2 |
| nitrogen dioxide | NO2 | 46.01 | X | -9.3 | 21.1 | X | X | 1.449 | X |
| penicillin G | C16H18N2O4S | 334.4 | benzylpenicillin | 217 | X | 1.4 | X | 1.69 | 8x103 |
| 11-cis-retinal |
|
284.44 | X | 64 | X | X | X | X | X |
| all trans-retinal |
|
284.44 | X | 61 | 421 | 0.948 | X | X | X |
| styrene | C8H8 | 104.15 | X | -31 | 145 | X | 0.909 | 1.516 | 2650 |
| 2,2,4-trimethylpentane | C8H18 | 113.13 | isooctane | 107.4 | 99 | X | 0.692 | 1.391 | 5000 |
| uranium -235 | U | 235.04 | X | ||||||
| uranium-238 | U | 238.08 | X | 1132 | 4132 | 18.7 | 750 | ||
| vanillin | C8H8O3 | 166.18 | X | 81 | 285 | 1.06 | X | 1.587 | 1580 |
I-B-2. The one on the left is water as ice floats in liquid water.
I-B-3. Since it is coldest at the top of the lake where the water is exposed to ambient temperature, the water would freeze at the surface but then sink as the ice would be denser than liquid water. This process would repeat until the lake filled with ice. This would make the lake very inhospitable to most life as we know it so we are fortunate that water behaves anomalously.
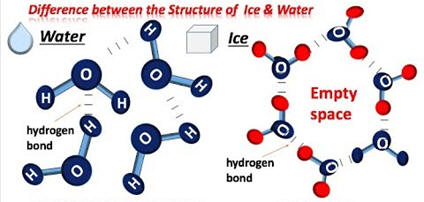
I-B-4. As can be observed in the images, hydrogen bonding results in ice having a very fixed structure the the water molecules farther apart than they are in liquid water.
I-C-1. Use of isooctane as the name for 2,2,4-trimethylpentane violates the rule for the meaning of "iso" and can lead to confusion and error.
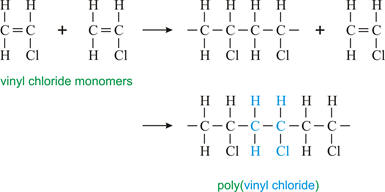 Uses:
pipes, medical devices, wire and cable insulation, window frames,
Uses:
pipes, medical devices, wire and cable insulation, window frames,
drainage pipe, water service pipe, medical devices, blood
storage bags, and many more.
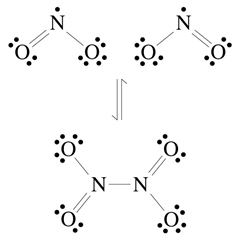 I-C-3-a. N2 + O2
= 2 NO 2 NO + O2
= 2 NO2
I-C-3-a. N2 + O2
= 2 NO 2 NO + O2
= 2 NO2
I-C-3-b. As the Lewis structure of NO2 correctly shows, NO2 is a free radical. There is a strongly energetic drive for free radicals to react and form a product with all paired electrons.
I-C-3-c. The free radicals are very reactive but when formal charges are added to the Lewis structure of N2O4 to the right, there are adjacent + charges on the nitrogen atoms and negative charges on two nearby oxygens. These repulsive forces destabilize the molecule and make the dimerization energetically close to neutral.
I-C-4-a. An -NH2 group
was added.
II-C-4-b. The amino group, -NH2 like ammonia is a
Brönsted
base and accepts protons in acidic media. The presence of the NH2
in amoxicillin (and ampicillin) enables the molecule to accept a proton in
the acidic stomach and become a relatively stable cation.
I-C-4-c. Any
change to a structure can affect the properties. It is like making a minor
change to a key. It probably will no longer fit the lock and work.
However, in the case of penicillin, the change apparently does not affect the
part of the molecule that fits correctly into the active site. Both
ampicillin and amoxicillin retain the antibiotic properties and for most
bacteria are even more effective than for penicillin G.
I-D-1. The melting and boiling points for heavy water are about 3.8 and 1.4 Co
higher. Since the atomic mass of D2O
is about 11% higher than for H2O, these differences
are not surprising. For isotopes of other elements, the
mass percent differences are much lower and most chemical and physical properties are
similar. Assuming the sizes of the two molecules are the same, the density
of D2O should be 11% higher than for H2O. This is
exactly what is observed. For hydrogen, the mass difference can result in significant rate
differences up to a factor of about seven if the breaking of a bond to the H or
D is involved in a rate determining step. For an example of the effect of
the isotope substitution on stretching frequencies, please see:
http://murov.info/orglab/50-x4.pdf
.
I-D-2.
Do you think it would be safe to drink heavy water?
A human can tolerate up to about 20% heavy water but a higher percentage can
cause problems with about 50% being lethal. As reaction rates change with
deuterium substitution, the biochemistry of the human body can be dangerously
altered by too much heavy water. see:
https://sciencenotes.org/can-you-drink-heavy-water-is-it-safe/
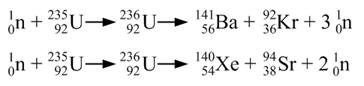
I-E-1. If a slight change is made to a key (e.g. 11-cis-retinal), the key will no longer open the lock. In this case, if 11-cis-retinal is isomerized by light, it will not longer fit in properly in the enzyme cavity and will be rejected from the site causing a signal to be sent to the vision center of the brain.
I-E-2. As can be observed, the values recorded in the table are within experimental
error, the same. However, about 90% of the population can distinguish
between the two isomers using odor differeences and the carvones rotate the
plane of polarized light in equal magnitudes but opposite directions.
| Compound | oral LD50 value for rats (mg/kg) | extrapolated amount for 70 kg human (g) |
| acetaminophen | 1944 | 136 |
| dioxin | 0.02 | 0.0014 |
| vanillin | 1580 | 111 |
I-F-2. 111 g or about 4 oz is much more than the approximately 5 g of vanillin in a
whole cake. This affords a large margin of safety.
I-F-3. 136/0.5 = 2.7x102 pills
I-F-4. 1.4 mg of dioxin can be toxic but paper products have about 100 ppt (parts per trillion) and weigh about 8 grams. This means the average plate has about 8x10-10 grams of dioxin or about 1 millionth of the amount toxic amount.
I-F-5. See: https://en.wikipedia.org/wiki/Pathophysiology_of_spider_bites
1.4x10-4 mL or 0.14 mg could be lethal for spiders and 3.5x10-2
mL or 35 mg for
snakes. For snakes the average amount of venom injected covers a huge
range with 50 mg about average whereas a black widow injects about 3x10-5
mL or about 0.03 mg. This calculation assumes humans have the same sensitivity
as rats. Also, there is a huge margin of error in all of the measurements
involved. The 0.03 mg injected by spiders is dangerous but not enough to kill
most people. However, 50 mg of snake venom is approximately the LD50 for
snakes and could result in death for about half the bites.
II. Identification of substances from properties.
| # | m.p. (oC) | b.p. (oC) | refractive index | IR info | Possible Cmpds. | Suggestions |
| 1 | 115 | 1.4100 | -OH, -CH3 | 3-methyl-2-butanol, 3-pentanol, 2-pentanol | nmr | |
| 2 | 122 | -OH, C=O | benzoic acid | |||
| 3 | 154 | 1.5160 | arom., -CH3 | anisole, allylbenzene | ir or nmr | |
| 4 | 202 | 1.5325 | C=O, -CH3 | acetophenone | ||
| 5 | 81 | arom., -CH3 | 1,2,4,5-tetramethylbenzene | |||
| 6 | 78 | 1.373 | -CH3, C=O | methyl propionate, ethyl acetate | nmr | |
| 7 | 114 | arom., C=O, -NH, -CH3 | acetanilide | |||
| 8 | 134 | 1.4585 | sp3 C-H | cyclohexylamine |
For more exercises with many spectra included, please visit:
http://murov.info/orglab/51-x5.pdf
http://www3.nd.edu/~smithgrp/structure/workbook.html
https://www.orgchemboulder.com/Spectroscopy/Problems/index.shtml
https://webspectra.chem.ucla.edu//
http://chemistry.miamioh.edu/organicspecid/
http://alpha.lasalle.edu/~price/202 COMBINED SPECTROSCOPY PROBLEMS.pdf
Note 1. During the preparation of this site, three interesting topics were encountered that did not seem to have adequate discussion during a limited Internet search.
1. Textbooks claim that except for hydrogen, isotopes have very similar properties but a comparison of physical and chemical properties of isotopes could not be located. Rate effects and nuclear effects are well documented but melting points and other physical properties of isotopes could not be found (e.g., U-235 and U-238).
2. The drug Austedo is used for the treatment of Huntington's disease. It contains deuterium atoms that apparently improve its efficacy. Although deuterium is an isotope of hydrogen, its properties are different enough that it could be considered to be a separate element but the best definitions of elements include the statement that elements have the same number of protons. This is a little like the demotion of Pluto from planet to dwarf planet.
3. Should there be a correlation between toxicity and color (visible region)?
4. A new discovery of a very strong plastic shows that the field of
material science is still in its early stages and has extensive potential
https://www.nature.com/articles/s41586-021-04296-3
,
hit counter started 2/21/22