
| Chemistry Teaching Momnents and Tips |  |
A selection of discussion topics for chemistry courses
that can be used to enhance, enrich and motivate learning. The list below
includes several examples that should stimulate interest and provide insight
into the topic. Accompanying each topic is a corresponding PowerPoint
slide that can be used to facilitate the discussion (http://murov.info/chemenrichment.pptx,
The second slide - Slide B - contains links to each slide by number and topic). Many of these topics
are included in a 33 page Chemistry Highlights web site that was
recently posted. http://murov.info/chemhighlights.htm
. Another Powerpoint website that provides similar topics is available at:
http://murov.info/chembeyondbook.htm For many more web sites by Steve Murov, please visit:
http://murov.info/.
For chemistry exercises, please visit:
http://murov.info/chemexe.htm
For information on periodic tables and ions, please visit:
http://murov.info/periodictables.htm
and http://murov.info/pertabtrends.htm
.
1. Safety First. Go to http://murov.info/chemenrichment.pptx, Slide 1.
2. Quotations by and about science. http://murov.info/chemenrichment.pptx, Slides 2a, 2b, 2c.
| 3. The story of the alchemists and their
search for the philosopher's stone along with their attempts to convert
lead into gold is an interesting prologue to the history of chemistry.
At least three of the paintings of alchemists are especially
interesting. A traditional painting of an alchemist by David
Teniers the Younger in a lab can be compared to a sketch by David
Teniers the Elder that has many similarities to the painting except that
the human alchemist has been replaced by an ape. The translation
of the French name of the sketch is Pleasure of Fools. An
ancestor of Teniers, Peter Bruegel created the engraving of an
alchemist's lab in disarray with the family apparently in the upper right
hand corner fleeing to a poor house as all their money had been
squandered on alchemistry. http://murov.info/chemenrichment.pptx, Slide 3 https://collections.artsmia.org/art/9645/the-alchemist-philips-galle |
 |
 |
 |
4. Observations. The first and key step in the scientific method is observation. Observation means more than use of the senses. A scientific observer notices if an observation is consistent with expectations. Two examples of discrepant events are described. http://murov.info/chemenrichment.pptx, Slides 4a, 4b.
a. Mass perception. Have students rank the mass of a ball bearing, rubber stopper and a cork ring by placing the object in the palm of his/her hand. Almost everyone will rank the ball bearing as the heaviest but if the appropriate size ball bearing (~20 g) is used, the cork ring will have the highest mass. Afterwards, have the students determine the mass of the three objects. There are a couple issues with this experiment. Most people expect the ball bearing to have the largest mass and the hand method probably measures pressure or mass/area rather than just mass. Students can be asked to devise a method using the hand that could give the correct ranking. For more information, see: http://murov.info/EM-st7.pdf and http://murov.info/chemenrichment.pptx, Slide 4c.
| b. The beaker in the figure on the right provides an example of the need for most of us to improve our observational skills. We all see ice in water frequently but how many ask if there is anything unusual about the observation. Should ice float in water? As a liquid cools, the volume should contract and since the mass stays the same, the density should increase. The density of the water should increase more upon freezing and the ice should sink. Therefore the observation of ice floating is not an adequate observation unless the question of why the ice is floating is asked. When asked about the beaker on the left and told that there is only one substance in the beaker, many students ignore the information provided and conclude that the liquid is water and the solid of another substance has sunk in the water. However, the liquid and solid are both acetophenone. Since people commonly see the ice-water system, they accept it as the norm. It is amazing that although millions of substances are known, water is probably the only substance that people have observed where they could tell if the solid would sink or float. Water is one of the very rare substances with a liquid phase denser than its solid. Gallium also exhibits this behavior. As expected, the density of water does increase as the temperature decreases down to 3.98oC but then unexpectedly slightly decreases until 0oC is reached. Upon freezing, there is about a 9% decrease in density with the result that ice floats. To demonstrate normal behavior, fill a test tube about half way with p-xylene (acetophenone works but has a stronger and annoying odor), insert in ice water for a short time and then scratch the inside of the test tube with a glass rod to induce partial solidification. As with almost all substances, the solid will go to the bottom. It is interesting to ask what the consequences would have been for life if water behave "normally". Also, why should a bottle not be filled to the top and capped and then frozen? For more information, see: http://murov.info/EM-st1.pdf and http://murov.info/chemenrichment.pptx, Slide 4d. |  |
| 5. Units and unit conversions. The true story of the Gimli Glider is one of several examples (for 10 examples, see: https://listverse.com/2019/08/30/10-simple-but-costly-math-errors-in-history/ ) that demonstrate the importance of units and unit conversions. A Boeing 767 on 7/23/1983 was scheduled to fly from Montreal to Edmonton with a stopover in Ottawa. However, due to faulty fuel gauges, a dip stick was used to measure the amount of gasoline already in the tank and a calculation was performed to determine the amount of gasoline that needed to be added. A unit conversion with incorrect units was used for the calculation and as a result instead of adding 20,000 liters, the ground crew only added about 5000 liters. After leaving Ottawa, the pilot noticed that there was insufficient fuel to make it to Edmonton and one engine was lost and eventually both engines were lost. Fortunately, the pilot had flown gliders and the co-pilot was aware of an old landing strip at Gimli near Winnipeg. The plane successfully landed among a group of people using the old landing strip although the nose gear collapsed and the nose veered into the runway. This might have been fortunate as this slowed up the plane so that it did not run into any of the people that had taken over the old landing strip to use it for cart races. Only minor injuries were experienced among the passengers when the emergency exit was not long enough to reach the ground due to the tilted plane. http://murov.info/chemenrichment.pptx, Slide 5a. |  |
Another very costly unit mistake caused the Mars Climate
Orbiter to be lost due to a mix up of metric and imperial units. For more
information on the Gimli Glider and the Mars Orbiter, please visit:
https://en.wikipedia.org/wiki/Gimli_Glider
,
https://www.cbc.ca/archives/when-a-metric-mix-up-led-to-the-gimli-glider-emergency-1.4754039
,
https://timeline.com/in-1983-two-pilots-miraculously-landed-a-jumbo-jet-with-no-fuel-from-40-000-feet-e51782deb01d
,
https://nationalpost.com/news/canada/gimli-glider-pilot-recalls-heroic-landing-of-air-canada-767-as-famed-plane-put-up-for-sale,
https://www.latimes.com/archives/la-xpm-1999-oct-01-mn-17288-story.html ,
http://edition.cnn.com/TECH/space/9909/30/mars.metric.02/
http://murov.info/chemenrichment.pptx,
Slide 5b.
6. The Periodic Table. For a timeline for the development of the periodic table, please visit: http://murov.info/timelines.htm . First, most people do not realize that only a small number of the elements are found on earth in elemental form. http://murov.info/chemenrichment.pptx, Slide 6a. The compilations of the atomic mass of the elements by Berzelius in 1828 and a much more complete and accurate version by Cannizaro in 1860 enabled several chemists to propose models to bring order to the properties of the approximately 60 elements know at the time. These efforts culminated in 1869 when Dmitri Mendeleev published the precursor of the modern periodic table. For a table that presents the elements in close to their colored form, see: http://murov.info/chemenrichment.pptx, Slide 6b. Today many variations exist (see: https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=All). Two of the common variations differ in the insertion point by one element of the lanthanides and actinides . The preferences have received substantial discussion in the literature especially by periodic table expert, Eric Scerri. Articles discussing the issues follow the periodic table figures. Both of these tables have the unique feature that the color of the box of each element is an attempt to represent the actual color of the element. http://murov.info/chemenrichment.pptx, Slide 6c.
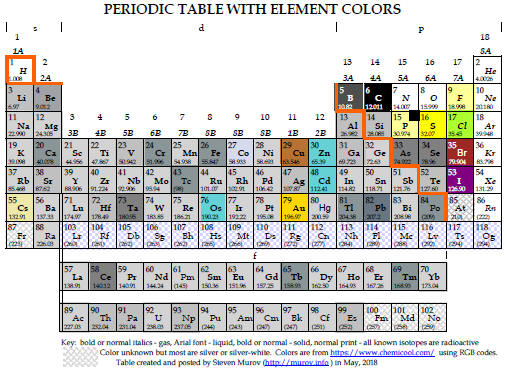 |
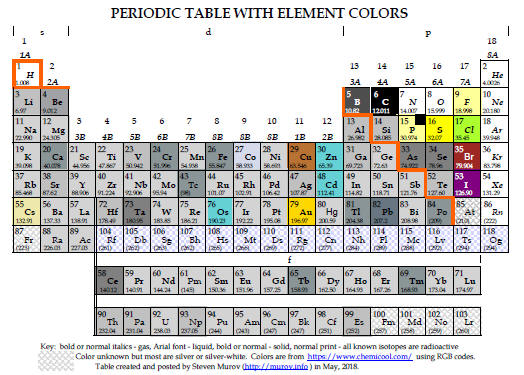 |
https://edu.rsc.org/feature/trouble-in-the-periodic-table/2020266.article
https://www.scientificamerican.com/article/the-evolution-of-the-periodic-system/
https://www.ifm.liu.se/edu/coursescms/NKEB06/lectures/grupp-3.pdf
https://www.academia.edu/1272686/Trouble_with_the_Periodic_Table
http://publications.iupac.org/ci/2012/3404/ud.html
https://cen.acs.org/physical-chemistry/periodic-table/periodic-table-icon-chemists-still/97/i1
7. Atomic mass and isotopes. The atomic mass was originally used by Mendeleev to order the elements. At that time, other than knowing that the atoms had some electrical properties, little if any was known about the composition of the atom and the relationships of the atoms. Did the atoms have any common features or were they all just different. In 1815, William Prout incorrectly but wisely suggested that all atoms were made of hydrogen atoms but it was not until decades later that it was discovered that protons, neutrons and electrons make up all the atoms with most of the mass of the atom concentrated in a nucleus made up of protons and neutrons. Moseley (about 1913) was able to show that the periodic table needed to be arranged in order of increasing number of protons (atomic number) instead of atomic mass. The value of the atomic mass is used to calculate the average number of neutrons in an atom (atomic mass - atomic number) and in mole calculations. The atomic mass contains more information than is commonly discussed and deserves more attention. For example, see: S. Murov, Chem 13 News, March, 2010. Promoting Insight: Atomic Mass. First, even before the existence of neutrons was verified by Chadwick in 1932, the atomic mass of helium (and other elements) provided evidence of the presence of neutrons in the helium nucleus as well as all of the elements with an atomic number of 2 or more. Periodic tables usually contain the symbol for the element, the atomic number and the atomic mass. Additional information is sometimes but not routinely included. Information about isotopes is seldom directly included but the atomic mass does provide some information about isotopes. If the atomic mass is within 0.1 of a whole number, there is a strong probability that there is a predominance of one isotope. If the atomic mass differs from unity by more than 0.1, there is a high probability that more than one stable isotope of the element exists. There are coincidences that lead to violations of these guidelines as bromine has an atomic mass of 79.904 g/mol but bromine 80 has a half life of 18 minutes and is not naturally occurring on earth. Naturally occurring bromine is about 50% bromine-79 and 50% bromine-81. It is also possible to make incorrect assumptions based on atomic mass. Chlorine has an atomic mass of 35.453 g/mol and one might assume that chlorine has two about equally abundant isomers, chlorine-35 and chlorine-36. However chlorine-36 has a half life of 300,000 years and naturally occurring stable isotopes are chlorine-35 (76%) and chlorine-37 (24%). To get a little more technical, stability of nuclei is favored by even numbers of protons and neutrons followed by even numbers of protons and even numbers of neutrons. The stable chlorine and bromine isotopes have even numbers of neutrons. A graph of the number of isotopes vs the atomic number supports these concepts. http://murov.info/chemenrichment.pptx, Slide 7a, 7b.
8. Mass and charge of protons, neutrons and electrons. Upon
inspection of the table below, does there seem to be any correlation between
mass and charge?
http://murov.info/chemenrichment.pptx,
Slide 8.
| Particle | Mass (g) | charge (coulombs) | Mass (amu) |
| proton | 1.6726×10−24 | 1.602176634×10−19 | 1.0072766 |
| neutron | 1.6749×10−24 | 0 | 1.0086654 |
| electron | 9.1094×10−28 | 1.602176634×10−19 | 0.0005486 |
9. Electron structure provides some insight into the shape of the periodic table. However, students often do not grasp the meaning of the symbols. Ask students to give the n, l, m, s values for a 3 p electron or ask for the symbol for an electron with n, l, m, s values of 4, 2, 1, 1/2. http://murov.info/chemenrichment.pptx, Slide 9.
10. The mole was recently redefined as 6.02214076×1023 units or 602.2 sextillion units (Avogadro's number). This number is too large to be easily imaginable. A small glass of water has ten times this number of molecules of water or 6 septillion molecules. Several attempts have been made to help make the magnitude of Avogadro's number imaginable. One example is would a mole of marshmallows (regular size) cover the surface area of the United States and if so, to what depth. Since a marshmallow is about 1 in3 and the surface area of the U.S. is 3.796x106 mi2, a mole of marshmallows would cover the U.S. to a depth of 623 miles. http://ths.sad44.org/ourpages/auto/2016/8/30/46345068/Mole%20fun%20facts%20_1_.pdf . It might be useful to ask students how many molecules of water would be present in 1x10-5 grams of water and if it would be possible on even the best balance available to weigh less than this number of molecules. This calculation helps to impress on students the need for a large unit (the mole) for chemical calculations. http://murov.info/chemenrichment.pptx, Slide 10b.
One example that demonstrates the lack of understanding of the mole involves a simple calculation. Students can usually determine molecular mass using the periodic table but when presented with a problem that gives the number of moles in a weighed amount of sample, many do not know how to determine the molecular mass. Another troubling problem of this type is the calculation of the molarity of water when assuming the density is 1.0 g/mL. The value also provides some insight as 55 M is about as concentrated as any solution can get. This provides an upper limit to molarity. Any calculation that gives a higher value should immediately be rechecked.
11. Oxidation numbers, formal charges and electronegativity provide useful indicators of bond information, reactivity and methods for keeping track of electrons and offer insight that needs to be part of an education in chemistry. Oxidation numbers assume that shared electrons are assigned to the more electronegative element. This results in an overestimate of the charge on the participating atoms but does facilitate an understanding of electron transfer in reactions. Formal charges divide shared electrons equally between the two partners resulting in an underestimate of the partial charges for atoms in bonds with atoms of different electronegativities. Calculated oxidation numbers that differ from expectations serve as an alert that some unusual chemical reactivity can be expected and even predicted. Non-zero formal charges also often lead to the expectation or understanding of chemical behavior. For a more complete discussion of these topics, please visit: http://murov.info/chemistryinsight.htm.
Oxidation number. For ions such as permanganate, chromate, chlorate, perchlorate, and nitrate, oxidation number calculations result in high positive numbers for the central atom. This is especially noteworthy for the very electronegative elements, chlorine and nitrogen. A consideration of the oxidation number should provide the insight that these ions could and probably will function as oxidizing agents. There are exceptions such as perchlorate but this can lead to a discussion of thermodynamic vs kinetic control. Unusual oxidation numbers for oxygen in hydrogen peroxide and hydrogen in hydrides provide correct alerts of high chemical reactivity. The oxidation numbers of the irons in Fe3O4 again provide an alert for unusual behavior albeit probably not the very unusual property for a compound of ferromagnetism. http://murov.info/chemenrichment.pptx, Slides 11a, 11b, 11c.
Formal charges. Unusual behavior for carbon monoxide and ozone are examples with formal charges that provide hints that unusual behavior can be be expected. This type of insight needs to be included when formal charge is discussed. Students should recognize that free radicals such as NO2 are expected to be reactive and dangerous when present in smog. It should also be noted that NO2 does dimerize as a result but the equilibrium between NO2 and N2O4 is not lopsided probably because of the interaction of the negative formal charges on the oxygens that contribute to instability. http://murov.info/chemenrichment.pptx, Slide 11d.
Electronegativity. Attempted modifications of the electronegativity compilations by Pauling have not solved empirical problems associated with the use of electronegativites for understanding and prediction. The carbon-iodine bond acts a polar bond with a partial positive charge on the carbon. Nucleophiles attack the carbon and displace a leaving group. Electronegativity values lead to the conclusion that the carbon iodine bond should be non-polar. Nitrogen and chlorine have about the same electronegativity but nitrogen participates in hydrogen bonding but chlorine does not. This is probably a size issue but students are sometimes given too much confidence in the value of electronegativity. Guidelines are often provided to enable students to determine if a bond is ionic or covalent. It is probably better to simplify by classifying metal to non-metal or polyatomic as ionic and non-metal to non-metal as covalent. Additionally, bonds between two different non-metals except for a carbon-hydrogen bond should be considered to be polar covalent. Bonds between the same two elements or carbon-hydrogen should be considered non-polar covalent. http://murov.info/chemenrichment.pptx, Slides 11f, 11g.
Molecular polarity. Electronegativities and formal charges are very useful when used with molecular geometry considerations for the prediction of molecular polarity. The polar bonds of water combined with the bent structure lead to the correct conclusion that water should be very polar. I2 with a non-polar bond is non-polar. CO2 has polar bonds but the dipoles for the two bonds cancel each other as a result of the linear geometry. Both are predicted to have low solubility in water (0.03% and 0.15% respectively). One guideline for solubility is 2%. If students observe a saturated iodine solution, it is possible to conclude that the prediction was wrong. Due to the high extinction coefficient of iodine, a small amount causes substantial color. CO2 escapes from an opened soda because of low solubility. Both predictions of low solubility were correct. http://murov.info/chemenrichment.pptx, Slides 11e, 11h.
12. pH. pH is a concept that most people have heard of and possibly even measured (e.g., a swimming pool) but few understand the meaning except that it is related to acidity. When asked to define pH, very few students if any will respond with the negative of the logarithm to the base 10 of the hydrogen ion concentration. As a result, when told that neutral water has a pH of 7, few realize how low the concentration of acid and base are in the solution. 1x10-7 M is so low that it is worth the time to ask students how they would prepare 100 mL of a 1x10-7 M NaCl solution. Calculations will show that this requires the weighing of about 6x10-7 g of NaCl. We learned previously that the limits of the best balances are 1x10-5 grams rendering the answer useless. What are the alternatives? A much larger volume could be prepared or about 10 Liters and the molarity would be very inaccurate as the weighing would be pushing the limits of the balance. Another option would be to make a more concentrated solution and then dilute but the final molarity would be subject to substantial error. These calculations give a sense of the magnitude of the hydrogen and hydroxide ion concentrations in a neutral water solution. It is also valuable to calculate the final pH of 1 L of neutral water if 1 drop of 1 M HCl is added. Also important for students to notice is that since pH is a logarithmic scale, each pH change of 1 unit is a 10 fold change of acidity. Finally, if students measure the pH of distilled water that has been sitting exposed to the air, they need to understand why the pH is about 6 instead of 7 and realize that it does not take much CO2 to cause this 1 pH unit change. http://murov.info/chemenrichment.pptx, Slides 12a, 12b.
13. The use of relevant topics to motivate. Explosives and penicillin would seem to have little in common but the structures of both can garner interest in students and increase motivation. If a slide of common explosives is shown, the students can be asked what, if any thing, the structures of the explosives have in common. Most explosives contain nitrogen and the reason for this can stimulate discussion on thermodynamics and molecular stability including the nitrogen triple bond. Explosives generally have highly exothermic reaction pathways that lead to production of huge volumes of gas. If the reaction is very fast, an explosion is possible. Incorporation of the development of the Haber-Bosch process and the relationship of the process to the needs of Germany during WWI can also be used to supplement this discussion. http://murov.info/chemenrichment.pptx, Slides 13a, 13b.
Penicillin G was serendipitously discovered by Alexander
Fleming in 1928. Despite his efforts, methods of delivery were not
discovered until the necessity caused by WWII infections resulted in the
development of methods for administering penicillin by Florey and Chain.
Because penicillin does not effectively survive stomach acid and had to be
injected, methods for chemically modifying penicillin G to make it stable in
acid were explored. This was accomplished by introducing an NH2 group into
the molecule. The protonation of ampicillin and amoxicillin results in the
formation of an ion that survives in stomach acid. This classic example of
drug design illustrated the use of basic chemistry concepts to solve an
important problem. Chemists and pharmacologists have also tried to modify
morphine to keep the strong analgesic properties while eliminating the addictive
property. Many of these attempts have resulted in commercially available
drugs but all are addictive and very dangerous if abused. It is likely
that analgesic and addictive properties are too closely connected in morphine
derivatives to be isolated or separated.
http://murov.info/chemenrichment.pptx,
Slides 13c, 13d.
Titanium is an interesting element that also deserves some attention. With a density much lower that steel but comparable strength, the question is why titanium is not used more. In addition, titanium's properties are not as sensitive to temperature as most metals are and it is resistant to interior oxidation. The primary reason is not abundance as it is abundant but the very expensive refining process. A reasonably priced refining process will result in much more titanium use and fame and fortune for the developer. http://murov.info/chemenrichment.pptx, Slide 13e.
14. Organic chemistry and isomers. The broad
and very important field of organic chemistry deserves more attention than it
will be given here. It is useful to look at the history of the definition
of organic chemistry. Until 1828, organic chemistry was the chemistry of
compounds that possessed a quality called vitalism that was claimed to be
derived from the living. Wohler was able to synthesize urea from
substances considered to have inanimate origins. Like all accepted ideas
it took many years before the concept of vitalism was abandoned with the new
definition of organic chemistry being the chemistry of carbon containing
compounds. Of particular interest in organic chemistry is the concept that
compounds can have the same formula but differ in structure, geometry or spatial
configuration. There are many isomers of octane, C8H18,
and it is interesting to note that one of them, 2,2,4-trimethylpentane, is the
basis for the octane rating of gas. This compound which has a commercial
name of isooctane but does not have the structure of isooctane according to
naming rules, has an octane rating of 100. Normal heptane has an octane
rating of 0. A gasoline mixture that knocks like a 90:10 ratio of
isooctane to heptane is given an octane rating of 90.
https://en.wikipedia.org/wiki/Octane_rating
.
http://murov.info/chemenrichment.pptx,
Slide 14a.
The geometric isomerism of cis-retinal to trans-retinal is caused by light and sends a signal to the brain that enables vision. Two of the most important classes of compounds needed for consumption are amino acids and sugars. Except for gylcine, all a amino acids have 4 different groups attached to the a carbon. These compounds all have non-superimposable mirror images. Almost all of the a amino acids found on earth have one of the configurations (L) and not the other. A person who consumed the D forms only would die of starvation because of the lack of the enzymes that metabolize the D isomers. Sugars have many carbons with 4 groups attached giving rise to many stereoisomers. For metabolic purposes on earth, only the sugars with the D configuration at the next to last carbon are commonly found in food. Mirror images differ in two primary ways: they rotate the plane of polarized in equal but opposite direction but more importantly interact with other stereoisomers in different ways. For instance, one set of amino acids in metabolized and the other is not. The compounds responsible for the odors of spearmint and caraway are non-superimposable mirror images of carvone. Because the receptors in our noses also have stereoisomeric sites, caraway smells different than spearmint to us. http://murov.info/chemenrichment.pptx, Slide 14b.
Because organic chemistry courses require the learning
and understanding of at least 100 reactions, a reaction map of organic chemistry
has been developed and posted that includes most of the reactions.
http://murov.info/Reaction-Map.htm.
Another site contains many exercises that use the reaction-map
http://murov.info/orgchemrxnexe.htm.
For many problems designed to provide insight into organic chemistry, please
visit the exercises at the back of the online organic laboratory text at:
http://murov.info/orglab.htm . The
exercises are also available at:
http://murov.info/orglab/exercises.htm
http://murov.info/chemenrichment.pptx,
Slide 14c.
15. Nuclear Chemistry. The periodic table contains useful information about nuclear stability. Most tables use some kind of symbolism to distinguish the elements that do not have stable isotopes although some elements that have only isotopes with very long half lives are not distinguished from the stable elements. All elements with atomic number equal to 83 (bismuth) or higher are to some extent radioactive. Technetium is the element with the lowest atomic number that does not have any naturally occurring stable isotopes but Tc is worthy of discussion because of its value as a radioactive tracer in medical analysis. http://murov.info/chemenrichment.pptx, Slide 15a. Uranium has an atomic mass of 238.03 which indicates that one isotope (238) predominates in nature. In the late 1930's it was discovered that the impact of a neutron with a U-235 nucleus results in fission but not with U-238. Because naturally occurring uranium is composed of 99.3% U-238, and 0.7% U-235, a sustained fission reaction requires concentrating the U-235. Because isotopes have almost the same chemical and physical properties, separating or even concentrating the lighter isotope is a tedious and expensive process. Oak Ridge in Tennessee was constructed during WWII for the purpose of enriching uranium with U-235. Success was achieved that led to the development of nuclear energy plants and nuclear weapons. The recent controversial nuclear arms treaty with Iran had to do with the facilities for enriching uranium. It should be noted that although U-238 does not fission when impacted by a neutron, it is converted to Pu-239 which does undergo fission. One of the bombs dropped on Japan in 1945 was a U-235 bomb and the other was a Pu-239 bomb. http://murov.info/chemenrichment.pptx, Slide 15b.
All elements with an atomic number above 92 (uranium) have to be synthesized and none are found to occur naturally (small amounts of Pt have apparently been recently discovered). As the atomic number increases, generally, the half life decreases. Nuclear stability calculations are difficult and complex but have concluded that around atomic number 114, it is possible that some stable or at least longer lives isotopes exist. Isotopes of these elements have been made but not the ones with the proton to neutron ratio that is predicted to have higher stability. http://murov.info/chemenrichment.pptx, Slide 15c.
16. Climate change. There is no doubt that as a result of fossil fuel burning by humans, the global climate is changing in ways that are harmful to many life forms on earth including humans. The global temperature record, sea level rise, ice mass loss, frequency and severity of damaging weather events provides indisputable evidence that we are on a pathway that will lead to many extinctions and sickness and death to humans. Despite the verified threat, many people including leaders are in denial and are not responding in ways that could minimize the damage. Some leaders have even lowered and even removed regulations that were passed to lower pollution levels. These actions will hasten and increase the damage of climate change. Already, the health of thousands is being negatively affected with many deaths that can be attributed to climate change and the rate of animal extinctions is increasing.
Part of the problem is a lack of knowledge of the issue
with a lack of climate change education in our school systems a major part of
the problem. Climate change education is addressed elsewhere
http://murov.info/climatechangeprobe.htm
but two PowerPoint slides have been included to emphasize two points. If
people are asked to name the 4 most abundant gases in dry air, very few know the
answers. Most do not know that nitrogen is the most abundant gas and an
extremely low number know that argon is number 3. Most include carbon
dioxide among the top 3 because they know plants need it and animals exhale it.
However, if asked what gases most people exhale, again most people name only
carbon dioxide even though it is only 3% of our exhaled gases. They do not
stop to think that we breathe in 78% nitrogen and then exhale the nitrogen.
Since the start of the industrial revolution, carbon dioxide has risen from 280
ppm to about 415 ppm. When the amount is given in ppm, most people do not
relate to the units or realize how small these numbers are. These ppm
numbers convert to an increase of 0.028% to 0.041% or an amount that will soon
represent a 50% increase. It needs to be pointed out that the low value of
0.041% is the reason that human activity has been able to change the composition
of the atmosphere. Oxygen is close to about 21% of the atmosphere and
human activity has a negligible effect on the oxygen content of the atmosphere.
Carbon dioxide plays a significant role in determining the global temperature.
By increasing the carbon dioxide, we are trapping more outgoing energy.
Carbon dioxide concentrations show a remarkable
correlation with increasing global temperatures. Some deniers argue that
the carbon dioxide is increasing because the temperature is increasing. It
is true that the correlation does not prove cause and effect. However, the
amount of carbon dioxide produced by human burning also correlates with the
carbon dioxide atmospheric increase and the temperature increase providing a
smoking gun.
http://murov.info/chemenrichment.pptx,
Slides 16a, 16b, 16c.
17. Fictional elements. On a lighter topic,
please refer to the website on fictional elements.
http://murov.info/fictionalelements.htm
http://murov.info/chemenrichment.pptx,
Slide 17a, 17b, 17c.
18. Check and recheck results - Does your answer make sense? Too often, students obtain results and then move on without questioning the logic of the result and its implications. As a carefully selected example, when students are asked to calculate the mass of an antimony atom, some will come out with a result of 7.3x1025 g because of the inverted use of Avogadro’s number. Making this mistake is ok but it is not ok to not notice that the answer is unreasonable as it is close to the mass of the moon. Calculations should always be followed by the question: Does the answer make sense? For example, values of atomic and molecular mass have boundary values and answers out of the logical range should lead to rechecking of the problem. The simple technique of using insight to determine if the result makes sense can frequently lead to a discovery of a calculation error. As instructors, we are also often too lax when it comes to encouraging students to learn from their mistakes. Students need to be encouraged and perhaps required to correct missed problems on tests. Students tend to merely look at the score and not learn from their mistakes. Probably more important than a tool for assessing progress in a course, tests should function as learning instruments. Insight gained from missed problems can go a long way toward making the student more competent in chemistry. When students realize they have acquired insight in the field of chemistry, they will become the positive learners that you will enjoy teaching in your classes. http://murov.info/chemenrichment.pptx, Slide 18.
Site uploaded by Steven Murov, see http://murov.info Comments and suggestions will be appreciated at murovs@yosemite.edu